Site-specific binding of a PPR protein defines and stabilizes 5' and 3' mRNA termini in chloroplasts
- PMID: 19424177
- PMCID: PMC2718276
- DOI: 10.1038/emboj.2009.121
Site-specific binding of a PPR protein defines and stabilizes 5' and 3' mRNA termini in chloroplasts
Abstract
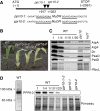
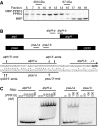
Chloroplast mRNA populations are characterized by overlapping transcripts derived by processing from polycistronic precursors. The mechanisms and functional significance of these processing events are poorly understood. We describe a pentatricopeptide repeat (PPR) protein, PPR10, whose binding defines mRNA segments derived from two transcription units in maize chloroplasts. PPR10 interacts in vivo and in vitro with two intergenic RNA regions of similar sequence. The processed 5' and 3' RNA termini in these regions overlap by approximately 25 nucleotides. The PPR10-binding sites map precisely to these overlapping sequences, and PPR10 is required specifically for the accumulation of RNAs with these termini. These findings show that PPR10 serves as a barrier to RNA decay from either the 5' or 3' direction and that a bound protein provides an alternative to an RNA hairpin as a barrier to 3' exonucleases. The results imply that protein 'caps' at both 5' and 3' ends can define the termini of chloroplast mRNA segments. These results, together with recent insights into bacterial RNA decay, suggest a unifying model for the biogenesis of chloroplast transcript populations and for the determinants of chloroplast mRNA stability.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Comment in
-
5' and 3' ends of chloroplast transcripts can both be stabilised by protein 'caps': a new model for polycistronic RNA maturation.EMBO J. 2009 Jul 22;28(14):1989-90. doi: 10.1038/emboj.2009.133. EMBO J. 2009. PMID: 19623192 Free PMC article. No abstract available.
References
-
- Barkan A (1998) Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol 297: 38–57
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

