Hippocampal lesions impair rapid learning of a continuous spatial alternation task
- PMID: 19424438
- PMCID: PMC2674562
- DOI: 10.1371/journal.pone.0005494
Hippocampal lesions impair rapid learning of a continuous spatial alternation task
Abstract
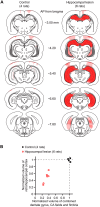
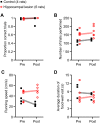
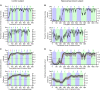
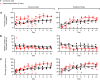
The hippocampus is essential for the formation of memories for events, but the specific features of hippocampal neural activity that support memory formation are not yet understood. The ideal experiment to explore this issue would be to monitor changes in hippocampal neural coding throughout the entire learning process, as subjects acquire and use new episodic memories to guide behavior. Unfortunately, it is not clear whether established hippocampally-dependent learning paradigms are suitable for this kind of experiment. The goal of this study was to determine whether learning of the W-track continuous alternation task depends on the hippocampal formation. We tested six rats with NMDA lesions of the hippocampal formation and four sham-operated controls. Compared to controls, rats with hippocampal lesions made a significantly higher proportion of errors and took significantly longer to reach learning criterion. The effect of hippocampal lesion was not due to a deficit in locomotion or motivation, because rats with hippocampal lesions ran well on a linear track for food reward. Rats with hippocampal lesions also exhibited a pattern of perseverative errors during early task experience suggestive of an inability to suppress behaviors learned during pretraining on a linear track. Our findings establish the W-track continuous alternation task as a hippocampally-dependent learning paradigm which may be useful for identifying changes in the neural representation of spatial sequences and reward contingencies as rats learn and apply new task rules.
Conflict of interest statement
Figures

References
-
- Amaral DG, Witter MP. Hippocampal Formation. In: Paxinos C, editor. The Rat Nervous System. Academic Press; 1995. pp. 443–493.
-
- Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia. Neurocase. 2001;7:357–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

