Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art
- PMID: 19425198
- PMCID: PMC2768545
- DOI: 10.1016/j.antiviral.2009.01.005
Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art
Abstract
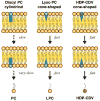
Although the acyclic nucleoside phosphonates cidofovir, adefovir and tenofovir are approved for treating human cytomegalovirus, hepatitis B and HIV infections, respectively, their utility is limited by low oral bioavailability, renal toxicity and poor cell penetration. Research over the past decade has shown that these undesirable features can be eliminated by esterifying the compounds with an alkoxyalkyl group, in effect disguising them as lysophospholipids. In this modified form, the drugs are readily taken up in the gastrointestinal tract and have a prolonged circulation time in plasma. The active metabolite also has a long half life within cells, permitting infrequent dosing. Because these modified drugs are not recognized by the transport mechanisms that cause the accumulation of acyclic nucleoside phosphonates in renal tubular cells, they lack nephrotoxicity. Alkoxyalkyl esterification also markedly increases the in vitro antiviral activity of acyclic nucleoside phosphonates by improving their delivery into cells. For example, an alkoxyalkyl ester of cyclic-cidofovir, a less soluble compound, retains anti-CMV activity for 3 months following a single intravitreal injection. Two of these novel compounds, hexadecyloxypropyl-cidofovir (CMX001) and hexadecyloxypropyl-tenofovir (CMX157) are now in clinical development. This article focuses on the hexadecyloxypropyl and octadecyloxyethyl esters of cidofovir and (S)-HPMPA, describing their synthesis and the evaluation of their in vitro and in vivo activity against a range of orthopoxviruses, herpesviruses, adenoviruses and other double-stranded DNA viruses. The extension to other nucleoside phosphonate antivirals is highlighted, demonstrating that this novel approach can markedly improve the medicinal properties of these drugs.
Figures

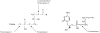


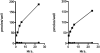
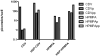
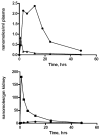


References
-
- Aldern KA, Ciesla SL, Winegarden KL, Hostetler KY. The increased antiviral activity of 1-O-hexadecyloxypropyl-cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol Pharmacol. 2003;63:678–681. - PubMed
-
- Aldern KA, Beadle JR, Hostetler KY. Comparison of the Intracellular Metabolism of Cidofovir and (S)-HPMPA and Their Hexadecyloxypropyl Esters. Antiviral Res. 2006;70:126, A57.
-
- Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D, De Clercq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethyoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–338. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

