VEGFR2+PDGFRbeta+ circulating precursor cells participate in capillary restoration after hyperoxia acute lung injury (HALI)
- PMID: 19426150
- PMCID: PMC2832073
- DOI: 10.1111/j.1582-4934.2009.00785.x
VEGFR2+PDGFRbeta+ circulating precursor cells participate in capillary restoration after hyperoxia acute lung injury (HALI)
Abstract
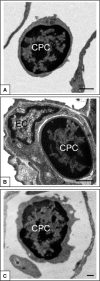
The in vivo morphology and phenotype of circulating cells that spontaneously contribute to new vessel formation in adults remain unclear. Here, we use high-resolution imaging and flow cytometry to characterize the morphology and phenotype of a distinct population of circulating mononuclear cells contributing to spontaneous new vessel formation after hyperoxia acute lung injury (HALI). We identify a subpopulation of myeloid (CD11b/Mac1(+)) haematopoietic cells co-expressing vascular endothelial growth factor receptor 2 (VEGFR2) and platelet derived growth factor receptor beta (PDGFRbeta). Moreover, we show that these CD11b(+)VEGFR2(+)PDGFRbeta(+) circulating precursor cells (CPCs) contribute structurally to the luminal surface of capillaries re-forming 2 weeks post-HALI. This indicates that these myeloid CPCs may function, at least transiently, as putative vascular precursors, and has important implications for capillary growth and repair in injury and in pathologies of the lung and other organs.
Figures





References
-
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. - PubMed
-
- Rafii S, Lyden D, Benezra R, et al. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy. Nat Rev Cancer. 2002;2:826–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

