Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA
- PMID: 19426209
- PMCID: PMC5567987
- DOI: 10.1111/j.1365-2958.2009.06670.x
Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA
Abstract
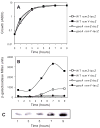
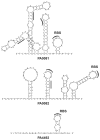
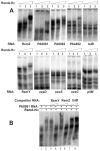
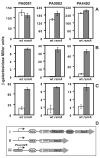
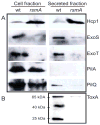
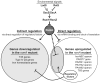
One of the prokaryotic post-transcriptional regulatory mechanisms involves the CsrA/RsmA family of proteins that act by modulating translation initiation at target mRNAs. In this study, we identified the regulon of RsmA of the Pseudomonas aeruginosa PAK strain by using cultures in the stationary phase of growth. The RsmA regulon includes over 500 genes, of which approximately one-third were affected by an rsmA mutation negatively, while the rest were affected positively. By isolating RsmA/mRNA complexes, analysing transcriptional and translational fusions, and performing gel-shift analyses, we identified 40 genes in six operons that are regulated by RsmA directly at the level of translation. All of these genes were affected by RsmA negatively and include genes encoding the type VI secretion system HSI-I, which has been implicated in the P. aeruginosa chronic infections. On the other hand, we were unable to demonstrate a direct interaction of RsmA with transcripts that are positively affected by this protein, including mRNAs encoding the type III secretion system and the type IV pili genes. Our work supports a model in which RsmA acts as a negative translational regulator, and where its positive effects are achieved indirectly by RsmA-mediated interference with translation of specific regulatory factors.
Figures


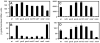
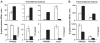

References
-
- Babitzke P, Romeo T. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr Opin Microbiol. 2007;10:156–163. - PubMed
-
- Baker CS, Morozov I, Suzuki K, Romeo T, Babitzke P. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol Microbiol. 2002;44:1599–1610. - PubMed
-
- Barnard FM, Loughlin MF, Fainberg HP, Messenger MP, Ussery DW, Williams P, Jenks PJ. Global regulation of virulence and the stress response by CsrA in the highly adapted human gastric pathogen Helicobacter pylori. Mol Microbiol. 2004;51:15–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

