CD28 and CTLA-4 coreceptor expression and signal transduction
- PMID: 19426212
- PMCID: PMC4186963
- DOI: 10.1111/j.1600-065X.2009.00770.x
CD28 and CTLA-4 coreceptor expression and signal transduction
Abstract
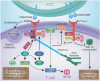
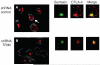
T-cell activation is mediated by antigen-specific signals from the TCRzeta/CD3 and CD4-CD8-p56lck complexes in combination with additional co-signals provided by coreceptors such as CD28, inducible costimulator (ICOS), cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death (PD-1), and others. CD28 and ICOS provide positive signals that promote and sustain T-cell responses, while CTLA-4 and PD-1 limit responses. The balance between stimulatory and inhibitory co-signals determines the ultimate nature of T-cell responses where response to foreign pathogen is achieved without excess inflammation and autoimmunity. In this review, we outline the current knowledge of the CD28 and CTLA-4 signaling mechanisms [involving phosphatidylinositol 3 kinase (PI3K), growth factor receptor-bound protein 2 (Grb2), Filamin A, protein kinase C theta (PKCtheta), and phosphatases] that control T-cell immunity. We also present recent findings on T-cell receptor-interacting molecule (TRIM) regulation of CTLA-4 surface expression, and a signaling pathway involving CTLA-4 activation of PI3K and protein kinase B (PKB)/AKT by which cell survival is ensured under conditions of anergy induction.
Figures



References
-
- Naluai A, et al. The CTLA4/CD28 gene region on chromosome 2q33 confers susceptibility to celiac disease in a way possibly distinct from that of type 1 diabetes and other chronic inflammatory disorders. Tissue Antigens. 2001;56:350–355. - PubMed
-
- Howard T, Rochelle J, Seldin M. Cd28 and Ctla-4, two related members of the Ig supergene family, are tightly linked on proximal mouse chromosome 1. Immunogenetics. 1991;33:74–76. - PubMed
-
- Balzano C, Buonavista N, Rouvier E, Golstein P. CTLA-4 and CD28: similar proteins, neighbouring genes. Int J Cancer Suppl. 1992;7:28–32. - PubMed
-
- Ikemizu S, et al. Structure and dimerization of a soluble form of B7-1. Immunity. 2000;12:51–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

