Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells
- PMID: 19426214
- PMCID: PMC2714548
- DOI: 10.1111/j.1600-065X.2009.00775.x
Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells
Abstract
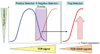
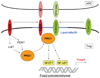
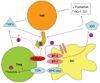
Costimulation is a concept that goes back to the early 1980s when Lafferty and others hypothesized that cell surface and soluble molecules must exist that are essential for initiating immune responses subsequent to antigen exposure. The explosion in this field of research ensued as over a dozen molecules have been identified to function as second signals following T-cell receptor engagement. By 1994, it seemed clear that the most prominent costimulatory pathway CD28 and functionally related costimulatory molecules, such as CD154, were the major drivers of a positive immune response. Then the immunology world turned upside down. CD28 knockout mice, which were, in most cases, immunodeficient, led to increased autoimmunity when bred into the non-obese diabetic background. Another CD28 family member, cytotoxic T-lymphocyte-associated protein 4, which was presumed to be a costimulatory molecule on activated T cells, turned out to be critical in downregulating immunity. These results, coupled with the vast suppressor cell literature which had been largely rebuked, suggested that the immune system was not poised for response but controlled in such a way that regulation was dominant. Over the last decade, we have learned that these costimulatory molecules play a key role in the now classical CD4(+)CD25(+)Foxp3(+) regulatory T cells (Tregs) that provide critical control of unwanted autoimmune responses. In this review, we discuss the connections between costimulation and Tregs that have changed the costimulation paradigm.
Figures
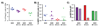
References
-
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. - PubMed
-
- Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. - PubMed
-
- Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. - PubMed
-
- Cheng MH, Shum AK, Anderson MS. What's new in the Aire? Trends Immunol. 2007;28:321–327. - PubMed
-
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

