The capsule of the fungal pathogen Cryptococcus neoformans
- PMID: 19426855
- PMCID: PMC2739887
- DOI: 10.1016/S0065-2164(09)01204-0
The capsule of the fungal pathogen Cryptococcus neoformans
Abstract
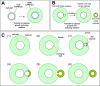
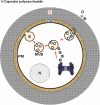
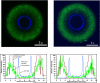
The capsule of the fungal pathogen Cryptococcus neoformans has been studied extensively in recent decades and a large body of information is now available to the scientific community. Well-known aspects of the capsule include its structure, antigenic properties and its function as a virulence factor. The capsule is composed primarily of two polysaccharides, glucuronoxylomannan (GXM) and galactoxylomannan (GalXM), in addition to a smaller proportion of mannoproteins (MPs). Most of the studies on the composition of the capsule have focused on GXM, which comprises more than 90% of the capsule's polysaccharide mass. It is GalXM, however, that is of particular scientific interest because of its immunological properties. The molecular structure of these polysaccharides is very complex and has not yet been fully elucidated. Both GXM and GalXM are high molecular mass polymers with the mass of GXM equaling roughly 10 times that of GalXM. Recent findings suggest, however, that the actual molecular weight might be different to what it has traditionally been thought to be. In addition to their structural roles in the polysaccharide capsule, these molecules have been associated with many deleterious effects on the immune response. Capsular components are therefore considered key virulence determinants in C. neoformans, which has motivated their use in vaccines and made them targets for monoclonal antibody treatments. In this review, we will provide an update on the current knowledge of the C. neoformans capsule, covering aspects related to its structure, synthesis and particularly, its role as a virulence factor.
Figures





References
-
- Aksenov SI, Babyeva IP, Golubev VI. On the mechanism of adaptation of micro-organisms to conditions of extreme low humidity. Life Sci Space Res. 1973;11:55–61. - PubMed
-
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16(21):2161–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

