A conserved Six-Eya cassette acts downstream of Wnt signaling to direct non-myogenic versus myogenic fates in the C. elegans postembryonic mesoderm
- PMID: 19427847
- PMCID: PMC2703692
- DOI: 10.1016/j.ydbio.2009.05.538
A conserved Six-Eya cassette acts downstream of Wnt signaling to direct non-myogenic versus myogenic fates in the C. elegans postembryonic mesoderm
Abstract
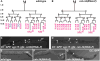
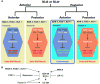
The subdivision of mesodermal cells into muscle and non-muscle cells is crucial to animal development. In the C. elegans postembryonic mesoderm, this subdivision is a result of an asymmetric cell division that leads to the formation of striated body wall muscles and non-muscle coelomocytes. Here we report that the Six homeodomain protein CEH-34 and its cofactor Eyes Absent, EYA-1, function synergistically to promote the non-muscle fate in cells also competent to form muscles. We further show that the asymmetric expression of ceh-34 and eya-1 is regulated by a combination of 1) mesodermal intrinsic factors MAB-5, HLH-1 and FOZI-1, 2) differential POP-1 (TCF/LEF) transcriptional activity along the anterior-posterior axis, and 3) coelomocyte competence factor(s). These factors are conserved in both vertebrates and invertebrates, suggesting a conserved paradigm for mesoderm development in metazoans.
Figures



References
-
- Amin NM, Hu K, Pruyne D, Terzic D, Bretscher A, Liu J. A Zn-finger/FH2-domain containing protein, FOZI-1, acts redundantly with CeMyoD to specify striated body wall muscle fates in the Caenorhabditis elegans postembryonic mesoderm. Development. 2007;1:19–29. - PubMed
-
- Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006;57:7505–7511. - PubMed
-
- Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell. 1997;7:893–903. - PubMed
-
- Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron. 1994;5:977–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

