Vaccinia virus strain differences in cell attachment and entry
- PMID: 19428041
- PMCID: PMC2700833
- DOI: 10.1016/j.virol.2009.04.012
Vaccinia virus strain differences in cell attachment and entry
Abstract
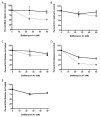
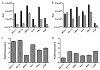
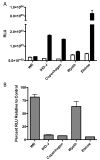
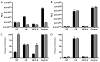
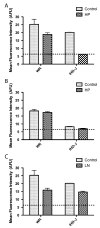
Vaccinia virus (VACV) strain WR can enter cells by a low pH endosomal pathway or direct fusion with the plasma membrane at neutral pH. Here, we compared attachment and entry of five VACV strains in six cell lines and discovered two major patterns. Only WR exhibited pH 5-enhanced rate of entry following neutral pH adsorption to cells, which correlated with sensitivity to bafilomycin A1, an inhibitor of endosomal acidification. Entry of IHD-J, Copenhagen and Elstree strains were neither accelerated by pH 5 treatment nor prevented by bafilomycin A1. Entry of the Wyeth strain, although not augmented by pH 5, was inhibited by bafilomycin A1. WR and Wyeth were both relatively resistant to the negative effects of heparin on entry, whereas the other strains were extremely sensitive due to inhibition of cell binding. The relative sensitivities of individual vaccinia virus strains to heparin correlated inversely with their abilities to bind to and enter glycosaminoglycan-deficient sog9 cells but not other cell lines tested. These results suggested that that IHD-J, Copenhagen and Elstree have a more limited ability than WR and Wyeth to use the low pH endosomal pathway and are more dependent on binding to glycosaminoglycans for cell attachment.
Figures




References
-
- Armstrong JA, Metz DH, Young MR. The mode of entry of vaccinia virus into L cells. J Gen Virol. 1973;21:533–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

