Emerging antiviral targets for influenza A virus
- PMID: 19428126
- PMCID: PMC2964876
- DOI: 10.1016/j.tips.2009.03.002
Emerging antiviral targets for influenza A virus
Abstract
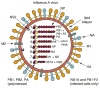
The potential threat of a pandemic caused by H5N1 influenza A viruses has stimulated increased research on developing new antivirals against influenza A viruses. Current antivirals are directed against the M2 protein (named adamantanes) and the neuraminidase (named zanamivir and oseltamivir). However, both seasonal and H5N1 influenza A viruses have developed resistance to adamantanes and oseltamivir. Accordingly, new antivirals directed at the M2 and neuraminidase proteins, and against the hemagglutinin protein, are being developed. In addition, elucidation of the structural basis for several crucial functions of other viral proteins (specifically the non-structural NS1A protein, the nucleoprotein and the viral polymerase) has identified novel targets for the development of new antivirals. Here, we describe how functional and structural studies led to the discovery of these novel targets and also how structural information is facilitating the rational design of new drugs against previously identified targets.
Figures
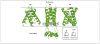




References
-
- Lamb RA, Krug RM. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Lippincott Williams & Wilkins; 2001. pp. 1487–1532.
-
- Wright PF, Webster RG. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Lippincott Williams & Wilkins; 2001. pp. 1533–1579.
-
- Reid AH, et al. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 2001;3:81–87. - PubMed
-
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. - PubMed
-
- Noah DL, Krug RM. Influenza virus virulence and its molecular determinants. In: Maramorsch K, Shatkin AJ, editors. Advances in Virus Research. Vol. 65. Elsevier; 2005. pp. 121–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

