Is complement good or bad for cancer patients? A new perspective on an old dilemma
- PMID: 19428302
- PMCID: PMC2704572
- DOI: 10.1016/j.it.2009.04.002
Is complement good or bad for cancer patients? A new perspective on an old dilemma
Abstract
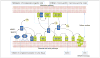
Several studies of human cancers have established that chronic and insidious inflammation promotes the process of carcinogenesis and exacerbates the growth of existing tumors. Conversely, acute inflammation seems to have the opposite effect. Recent discoveries indicate that this dualism in the role of inflammation in cancer is mirrored by the effects of the complement system on this disease process. Previous studies have suggested that complement proteins can contribute to the immune surveillance of malignant tumors. However, a very recent study has indicated that complement proteins can also promote tumor growth. Here, we describe our current understanding of the role of complement in tumor development and progression.
Figures

References
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. - PubMed
-
- Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64:529–564. - PubMed
-
- Dunn GP, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

