Quantitative evaluation of serotonin release and clearance in Drosophila
- PMID: 19428541
- PMCID: PMC2691387
- DOI: 10.1016/j.jneumeth.2009.02.013
Quantitative evaluation of serotonin release and clearance in Drosophila
Abstract
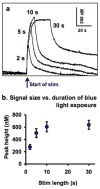
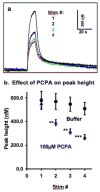
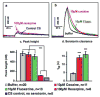
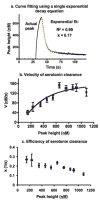
Serotonin signaling plays a key role in the regulation of development, mood and behavior. Drosophila is well suited for the study of the basic mechanisms of serotonergic signaling, but the small size of its nervous system has previously precluded the direct measurements of neurotransmitters. This study demonstrates the first real-time measurements of changes in extracellular monoamine concentrations in a single larval Drosophila ventral nerve cord. Channelrhodopsin-2-mediated, neuronal type-specific stimulation is used to elicit endogenous serotonin release, which is detected using fast-scan cyclic voltammetry at an implanted microelectrode. Release is decreased when serotonin synthesis or packaging are pharmacologically inhibited, confirming that the detected substance is serotonin. Similar to tetanus-evoked serotonin release in mammals, evoked serotonin concentrations are 280-640nM in the fly, depending on the stimulation length. Extracellular serotonin signaling is prolonged after administering cocaine or fluoxetine, showing that transport regulates the clearance of serotonin from the extracellular space. When ChR2 is targeted to dopaminergic neurons, dopamine release is measured demonstrating that this method is broadly applicable to other neurotransmitter systems. This study shows that the dynamics of serotonin release and reuptake in Drosophila are analogous to those in mammals, making this simple organism more useful for the study of the basic physiological mechanisms of serotonergic signaling.
Figures


References
-
- Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. - PubMed
-
- Bruns D, Jahn R. Real-time measurement of transmitter release from single synaptic vesicles. Nature. 1995;377:62–65. - PubMed
-
- Bruns D, Riedel D, Klingauf J, Jahn R. Quantal release of serotonin. Neuron. 2000;28:205–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

