Acetylcholine efflux from retrosplenial areas and hippocampal sectors during maze exploration
- PMID: 19428644
- PMCID: PMC2680767
- DOI: 10.1016/j.bbr.2009.02.023
Acetylcholine efflux from retrosplenial areas and hippocampal sectors during maze exploration
Abstract
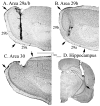
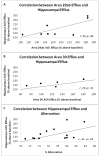
Both the retrosplenial cortex (RSC) and the hippocampus are important for spatial learning across species. Although hippocampal acetylcholine (ACh) release has been associated with learning on a number of spatial tasks, relatively little is understood about the functional role of ACh release in the RSC. In the present study, spatial exploration was assessed in rats using a plus maze spontaneous alternation task. ACh efflux was assessed simultaneously in the hippocampus and two sub-regions of the RSC (areas 29ab and 30) before, during and after maze exploration. Results demonstrated that there was a significant rise in ACh efflux in RSC area 29ab and the hippocampus during maze traversal. The rise in ACh efflux across these two regions was correlated. There were no significant behaviorally driven changes in ACh efflux in RSC area 30. While both the hippocampal sectors and area 29ab displayed increases in ACh efflux during maze exploration, the percent ACh rise in area 29ab was higher than that observed in the hippocampus and persisted into the post-baseline period. Joint efflux analyses demonstrated a key functional role for ACh release in area 29ab during spatial processing.
Figures

References
-
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal - anterior thalamic axis. Behav Brain Science. 1999;22:425–489. - PubMed
-
- Albasser MM, Poiriur GL, Warburton EC, Aggleton JP. Hippocampal lesions have-early gene protein counts in retrosplenial cortex: Distal dysfunction in spatial memory system. Eur J Neurosci. 2007;26:1254–1260. - PubMed
-
- Amaral DG, Kurz J. An analyses of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240:37–59. - PubMed
-
- Biegler R. Possible uses of path integration in animal navigation. Anim Learn Behav. 2000;28:257–277.
-
- Big IV, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Res. 1982;8:727–763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

