Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy
- PMID: 19428913
- PMCID: PMC2695996
- DOI: 10.1016/j.vaccine.2009.03.034
Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy
Abstract
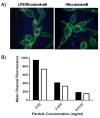
Innate immune system activation is a critical step in the initiation of an effective adaptive immune response; therefore, activation of a class of innate pathogen receptors called pattern recognition receptors (PRR) is a central feature of many adjuvant systems. It has recently been shown that one member of an intracellular PRR, the NLRP3 inflammasome, is activated by a number of classical adjuvants including aluminum hydroxide and saponins [Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008;453(June (7198)):1122-6; Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol 2008;181(July (1)):17-21]. Inflammasome activation in vitro requires signaling of both the Toll-like receptor (TLR) and NLRP3 in antigen-presenting cells. Here we present a class of nanomaterials endowed with these two signals for rapid optimization of vaccine design. We constructed this system using a simple approach that incorporates lipopolysaccharides (LPS) onto the surface of nanoparticles constructed from a biocompatible polyester, poly(lactic-co-glycolic acid) (PLGA), loaded with antigen. We demonstrate that LPS-modified particles are preferentially internalized by dendritic cells compared to uncoated nanoparticles and the system, when administered to mice, elicits potent humoral and cellular immunity against a model antigen, ovalbumin. Wild-type macrophages pulsed with LPS-modified nanoparticles resulted in production of the proinflammatory cytokine IL-1beta consistent with inflammasome activation. In comparison, NLRP3-deficient and caspase-1-deficient macrophages showed negligible production of IL-1beta. Furthermore, when endocytosis and lysosomal destabilization were inhibited, inflammasome activity was diminished, supporting the notion that nanoparticles rupture lysosomal compartments and behave as 'danger signals' [Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008;9(August (8)):847-56]. The generality of this vaccination approach is tested by encapsulation of a recombinant West Nile envelope protein and demonstrated by protection against a murine model of West Nile encephalitis. The design of such an antigen delivery mechanism with the ability to stimulate two potent innate immune pathways represents a potent new approach to simultaneous antigen and adjuvant delivery.
Figures

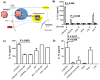
 ) and caspase-1-deficient (■) mice were isolated and incubated with LPS-modified and unmodified OVA-loaded particles with and without prior stimulation with soluble LPS. ATP with LPS pre-stimulation was used as a positive control. After 24 h, supernatant was analyzed for IL-1β by ELISA. C) WT macrophages and D) BMDCs were pre-stimulated with LPS and then treated with cytochalasin D (cytoD), to inhibit internalization; CA-074 Me, to inhibit cathepsin B; or media alone. After incubation with particles or alum for 24 h or ATP for 20 min, supernatant was analyzed for IL-1β.
) and caspase-1-deficient (■) mice were isolated and incubated with LPS-modified and unmodified OVA-loaded particles with and without prior stimulation with soluble LPS. ATP with LPS pre-stimulation was used as a positive control. After 24 h, supernatant was analyzed for IL-1β by ELISA. C) WT macrophages and D) BMDCs were pre-stimulated with LPS and then treated with cytochalasin D (cytoD), to inhibit internalization; CA-074 Me, to inhibit cathepsin B; or media alone. After incubation with particles or alum for 24 h or ATP for 20 min, supernatant was analyzed for IL-1β.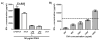
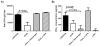

 ); LPS/rWNVE s.c. (
); LPS/rWNVE s.c. ( ); LPS/rWNVE oral (
); LPS/rWNVE oral ( ); and LPS/rWNVE nasal (
); and LPS/rWNVE nasal ( ;). Results are combined data from two experiments (n=10).
;). Results are combined data from two experiments (n=10).References
-
- Singh M, O’Hagan DT. Recent advances in vaccine adjuvants. Pharm Res. 2002 Jun;19(6):715–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

