A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali
- PMID: 19428923
- PMCID: PMC2713037
- DOI: 10.1016/j.vaccine.2009.03.014
A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali
Abstract
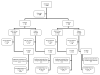
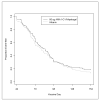
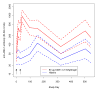
A double blind, randomized, controlled Phase 2 clinical trial was conducted to assess the safety, immunogenicity, and biologic impact of the vaccine candidate Apical Membrane Antigen 1-Combination 1 (AMA1-C1), adjuvanted with Alhydrogel. Participants were healthy children 2-3 years old living in or near the village of Bancoumana, Mali. A total of 300 children received either the study vaccine or the comparator. No impact of vaccination was seen on the primary endpoint, the frequency of parasitemia measured as episodes >3000/microL/day at risk. There was a negative impact of vaccination on the hemoglobin level during clinical malaria, and mean incidence of hemoglobin <8.5 g/dL, in the direction of lower hemoglobin in the children who received AMA1-C1, although these differences were not significant after correction for multiple tests. These differences were not seen in the second year of transmission.
Figures

References
-
- WHO. World Malaria Report. 2008. http://www.who.int/malaria/wmr2008/malaria2008.pdf.
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004 Oct 16 22;364(9443):1411–20. - PubMed
-
- Girard MP, Reed ZH, Friede M, Kieny MP. A review of human vaccine research and development: malaria. Vaccine. 2007;15(9):1567–80. - PubMed
-
- Epstein JE, Giersing B, Mullen G, Moorthy V, Richie TL. Malaria vaccines: are we getting closer? Curr Opin Mol Ther. 2007 Feb;9(1):11–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

