Nanoparticle-chelator conjugates as inhibitors of amyloid-beta aggregation and neurotoxicity: a novel therapeutic approach for Alzheimer disease
- PMID: 19429118
- PMCID: PMC2683427
- DOI: 10.1016/j.neulet.2009.03.064
Nanoparticle-chelator conjugates as inhibitors of amyloid-beta aggregation and neurotoxicity: a novel therapeutic approach for Alzheimer disease
Abstract
Oxidative stress and amyloid-beta are considered major etiological and pathological factors in the initiation and promotion of neurodegeneration in Alzheimer disease (AD). Insomuch as causes of such oxidative stress, transition metals, such as iron and copper, which are found in high concentrations in the brains of AD patients and accumulate specifically in the pathological lesions, are viewed as key contributors to the altered redox state. Likewise, the aggregation and toxicity of amyloid-beta is dependent upon transition metals. As such, chelating agents that selectively bind to and remove and/or "redox silence" transition metals have long been considered as attractive therapies for AD. However, the blood-brain barrier and neurotoxicity of many traditional metal chelators has limited their utility in AD or other neurodegenerative disorders. To circumvent this, we previously suggested that nanoparticles conjugated to iron chelators may have the potential to deliver chelators into the brain and overcome such issues as chelator bioavailability and toxic side-effects. In this study, we synthesized a prototype nanoparticle-chelator conjugate (Nano-N2PY) and demonstrated its ability to protect human cortical neurons from amyloid-beta-associated oxidative toxicity. Furthermore, Nano-N2PY nanoparticle-chelator conjugates effectively inhibited amyloid-beta aggregate formation. Overall, this study indicates that Nano-N2PY, or other nanoparticles conjugated to metal chelators, may provide a novel therapeutic strategy for AD and other neurodegenerative diseases associated with excess transition metals.
Figures
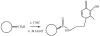


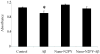

Similar articles
-
Chapter 5 - Development of iron chelator-nanoparticle conjugates as potential therapeutic agents for Alzheimer disease.Prog Brain Res. 2009;180:97-108. doi: 10.1016/S0079-6123(08)80005-2. Epub 2009 Dec 8. Prog Brain Res. 2009. PMID: 20302830 Review.
-
Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance.Neurosci Lett. 2006 Oct 9;406(3):189-93. doi: 10.1016/j.neulet.2006.07.020. Epub 2006 Aug 21. Neurosci Lett. 2006. PMID: 16919875
-
Nanoparticle and iron chelators as a potential novel Alzheimer therapy.Methods Mol Biol. 2010;610:123-44. doi: 10.1007/978-1-60327-029-8_8. Methods Mol Biol. 2010. PMID: 20013176 Free PMC article.
-
Metal Ions in Alzheimer's Disease: A Key Role or Not?Acc Chem Res. 2019 Jul 16;52(7):2026-2035. doi: 10.1021/acs.accounts.9b00248. Epub 2019 Jul 5. Acc Chem Res. 2019. PMID: 31274278
-
Development of beta-amyloid-induced neurodegeneration in Alzheimer's disease and novel neuroprotective strategies.Rev Neurosci. 2005;16(3):181-212. doi: 10.1515/revneuro.2005.16.3.181. Rev Neurosci. 2005. PMID: 16323560 Review.
Cited by
-
Designing and Formulation of Nanocarriers for "Alzheimer's and Parkinson's" Early Detection and Therapy.CNS Neurol Disord Drug Targets. 2024;23(10):1251-1262. doi: 10.2174/0118715273297024240201055550. CNS Neurol Disord Drug Targets. 2024. PMID: 38351689 Review.
-
Recent Status of Nanomaterial Fabrication and Their Potential Applications in Neurological Disease Management.Nanoscale Res Lett. 2018 Aug 10;13(1):231. doi: 10.1186/s11671-018-2638-7. Nanoscale Res Lett. 2018. PMID: 30097809 Free PMC article. Review.
-
Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases.Pharm Res. 2013 Oct;30(10):2499-511. doi: 10.1007/s11095-013-1156-7. Pharm Res. 2013. PMID: 23959851 Review.
-
Aggregation modulators interfere with membrane interactions of β2-microglobulin fibrils.Biophys J. 2013 Aug 6;105(3):745-55. doi: 10.1016/j.bpj.2013.06.015. Biophys J. 2013. PMID: 23931322 Free PMC article.
-
Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment.J Alzheimers Dis. 2010;19(1):363-72. doi: 10.3233/JAD-2010-1239. J Alzheimers Dis. 2010. PMID: 20061651 Free PMC article. Review.
References
-
- Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. - PubMed
-
- Atwood CS, Scarpa RC, Huang X, Moir RD, Jones WD, Fairlie DP, Tanzi RE, Bush AI. Characterization of copper interactions with alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta1-42. J Neurochem. 2000;75:1219–1233. - PubMed
-
- Behl C, Davis J, Cole GM, Schubert D. Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem Biophys Res Commun. 1992;186:944–950. - PubMed
-
- Bishop GM, Robinson SR, Liu Q, Perry G, Atwood CS, Smith MA. Iron: a pathological mediator of Alzheimer disease? Dev Neurosci. 2002;24:184–187. - PubMed
-
- Blake DR, Winyard P, Lunec J, Williams A, Good PA, Crewes SJ, Gutteridge JM, Rowley D, Halliwell B, Cornish A, et al. Cerebral and ocular toxicity induced by desferrioxamine. Q J Med. 1985;56:345–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

