Fermentation pH influences the physiological-state dynamics of Lactobacillus bulgaricus CFL1 during pH-controlled culture
- PMID: 19429565
- PMCID: PMC2704822
- DOI: 10.1128/AEM.02725-08
Fermentation pH influences the physiological-state dynamics of Lactobacillus bulgaricus CFL1 during pH-controlled culture
Abstract
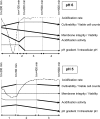
This study aims at better understanding the effects of fermentation pH and harvesting time on Lactobacillus bulgaricus CFL1 cellular state in order to improve knowledge of the dynamics of the physiological state and to better manage starter production. The Cinac system and multiparametric flow cytometry were used to characterize and compare the progress of the physiological events that occurred during pH 6 and pH 5 controlled cultures. Acidification activity, membrane damage, enzymatic activity, cellular depolarization, intracellular pH, and pH gradient were determined and compared during growing conditions. Strong differences in the time course of viability, membrane integrity, and acidification activity were displayed between pH 6 and pH 5 cultures. As a main result, the pH 5 control during fermentation allowed the cells to maintain a more robust physiological state, with high viability and stable acidification activity throughout growth, in opposition to a viability decrease and fluctuation of activity at pH 6. This result was mainly explained by differences in lactate concentration in the culture medium and in pH gradient value. The elevated content of the ionic lactate form at high pH values damaged membrane integrity that led to a viability decrease. In contrast, the high pH gradient observed throughout pH 5 cultures was associated with an increased energetic level that helped the cells maintain their physiological state. Such results may benefit industrial starter producers and fermented-product manufacturers by allowing them to better control the quality of their starters, before freezing or before using them for food fermentation.
Figures



References
-
- Adamberg, K., S. Kask, T. M. Laht, and T. Paalme. 2003. The effect of temperature and pH on the growth of lactic acid bacteria: a pH-auxostat study. Int. J. Food Microbiol. 85:171-183. - PubMed
-
- Amrane, A., and Y. Prigent. 1999. Differentiation of pH and free lactic acid effects on the various growth and production phases of Lactobacillus helveticus. J. Chem. Technol. Biot. 74:33-40.
-
- Barreto, M. T. O., E. P. Melo, J. S. Almeida, A. M. R. B. Xavier, and M. J. T. Carrondo. 1991. A kinetic method for calculating the viability of lactic starter cultures. Appl. Microbiol. Biotechnol. 34:648-652.
-
- Béal, C., and G. Corrieu. 1991. Influence of pH, temperature, and inoculum composition on mixed cultures of Streptococcus thermophilus 404 and Lactobacillus bulgaricus 398. Biotechnol. Bioeng. 38:90-98. - PubMed
-
- Béal, C., P. Louvet, and G. Corrieu. 1989. Influence of controlled pH and temperature on the growth and acidification of pure cultures of Streptococcus thermophilus 404 and Lactobacillus bulgaricus 398. Appl. Microbiol. Biotechnol. 32:148-154.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

