A 20-amino acid module of protein kinase C{epsilon} involved in translocation and selective targeting at cell-cell contacts
- PMID: 19429675
- PMCID: PMC2707237
- DOI: 10.1074/jbc.M109.004614
A 20-amino acid module of protein kinase C{epsilon} involved in translocation and selective targeting at cell-cell contacts
Abstract
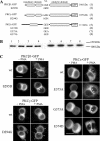
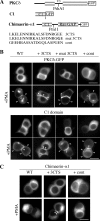
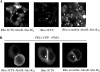
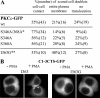
In the pituitary gland, activated protein kinase C (PKC) isoforms accumulate either selectively at the cell-cell contact (alpha and epsilon) or at the entire plasma membrane (beta1 and delta). The molecular mechanisms underlying these various subcellular locations are not known. Here, we demonstrate the existence within PKCepsilon of a cell-cell contact targeting sequence (3CTS) that, upon stimulation, is capable of targeting PKCdelta, chimerin-alpha1, and the PKCepsilon C1 domain to the cell-cell contact. We show that this selective targeting of PKCepsilon is lost upon overexpression of 3CTS fused to a (R-Ahx-R)(4) (where Ahx is 6-aminohexanoic acid) vectorization peptide, reflecting a dominant-negative effect of the overexpressed 3CTS on targeting selectivity. 3CTS contains a putative amphipathic alpha-helix, a 14-3-3-binding site, and the Glu-374 amino acid, involved in targeting selectivity. We show that the integrity of the alpha-helix is important for translocation but that 14-3-3 is not involved in targeting selectivity. However, PKCepsilon translocation is increased when PKCepsilon/14-3-3 interaction is abolished, suggesting that phorbol 12-myristate 13-acetate activation may initiate two sets of PKCepsilon functions, those depending on 14-3-3 and those depending on translocation to cell-cell contacts. Thus, 3CTS is involved in the modulation of translocation via its 14-3-3-binding site, in cytoplasmic desequestration via the alpha-helix, and in selective PKCepsilon targeting at the cell-cell contact via Glu-374.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

