Ceramide stimulates ABCA12 expression via peroxisome proliferator-activated receptor {delta} in human keratinocytes
- PMID: 19429679
- PMCID: PMC2707228
- DOI: 10.1074/jbc.M109.006973
Ceramide stimulates ABCA12 expression via peroxisome proliferator-activated receptor {delta} in human keratinocytes
Abstract
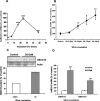
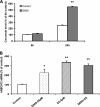
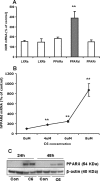
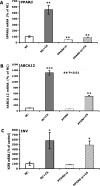
ABCA12 (ATP binding cassette transporter, family 12) is a cellular membrane transporter that facilitates the delivery of glucosylceramides to epidermal lamellar bodies in keratinocytes, a process that is critical for permeability barrier formation. Following secretion of lamellar bodies into the stratum corneum, glucosylceramides are metabolized to ceramides, which comprise approximately 50% of the lipid in stratum corneum. Gene mutations of ABCA12 underlie harlequin ichthyosis, a devastating skin disorder characterized by abnormal lamellar bodies and a severe barrier abnormality. Recently we reported that peroxisome proliferator-activated receptor (PPAR) and liver X receptor activators increase ABCA12 expression in human keratinocytes. Here we demonstrate that ceramide (C(2)-Cer and C(6)-Cer), but not C(8)-glucosylceramides, sphingosine, or ceramide 1-phosphate, increases ABCA12 mRNA expression in a dose- and time-dependent manner. Inhibitors of glucosylceramide synthase, sphingomyelin synthase, and ceramidase and small interfering RNA knockdown of human alkaline ceramidase, which all increase endogenous ceramide levels, also increased ABCA12 mRNA levels. Moreover, simultaneous treatment with C(6)-Cer and each of these same inhibitors additively increased ABCA12 expression, indicating that ceramide is an important inducer of ABCA12 expression and that the conversion of ceramide to other sphingolipids or metabolites is not required. Finally, both exogenous and endogenous ceramides preferentially stimulate PPARdelta expression (but not other PPARs or liver X receptors), whereas PPARdelta knockdown by siRNA transfection specifically diminished the ceramide-induced increase in ABCA12 mRNA levels, indicating that PPARdelta is a mediator of the ceramide effect. Together, these results show that ceramide, an important lipid component of epidermis, up-regulates ABCA12 expression via the PPARdelta-mediated signaling pathway, providing a substrate-driven, feed-forward mechanism for regulating this key lipid transporter.
Figures


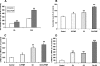


Similar articles
-
PPAR and LXR activators regulate ABCA12 expression in human keratinocytes.J Invest Dermatol. 2008 Jan;128(1):104-9. doi: 10.1038/sj.jid.5700944. Epub 2007 Jul 5. J Invest Dermatol. 2008. PMID: 17611579
-
The roles of ABCA12 in epidermal lipid barrier formation and keratinocyte differentiation.Biochim Biophys Acta. 2014 Mar;1841(3):435-40. doi: 10.1016/j.bbalip.2013.08.009. Epub 2013 Aug 15. Biochim Biophys Acta. 2014. PMID: 23954554 Review.
-
Defects in Stratum Corneum Desquamation Are the Predominant Effect of Impaired ABCA12 Function in a Novel Mouse Model of Harlequin Ichthyosis.PLoS One. 2016 Aug 23;11(8):e0161465. doi: 10.1371/journal.pone.0161465. eCollection 2016. PLoS One. 2016. PMID: 27551807 Free PMC article.
-
Endogenous β-glucocerebrosidase activity in Abca12⁻/⁻epidermis elevates ceramide levels after topical lipid application but does not restore barrier function.J Lipid Res. 2014 Mar;55(3):493-503. doi: 10.1194/jlr.M044941. Epub 2013 Nov 30. J Lipid Res. 2014. PMID: 24293640 Free PMC article.
-
Harlequin ichthyosis: ABCA12 mutations underlie defective lipid transport, reduced protease regulation and skin-barrier dysfunction.Cell Tissue Res. 2013 Feb;351(2):281-8. doi: 10.1007/s00441-012-1474-9. Epub 2012 Aug 4. Cell Tissue Res. 2013. PMID: 22864982 Review.
Cited by
-
Genetic pathways in disorders of epidermal differentiation.Trends Genet. 2013 Jan;29(1):31-40. doi: 10.1016/j.tig.2012.10.005. Epub 2012 Nov 8. Trends Genet. 2013. PMID: 23141808 Free PMC article. Review.
-
Molecular Mechanism of Epidermal Barrier Dysfunction as Primary Abnormalities.Int J Mol Sci. 2020 Feb 11;21(4):1194. doi: 10.3390/ijms21041194. Int J Mol Sci. 2020. PMID: 32054030 Free PMC article. Review.
-
Expression and regulation of GPAT isoforms in cultured human keratinocytes and rodent epidermis.J Lipid Res. 2010 Nov;51(11):3207-16. doi: 10.1194/jlr.M007054. Epub 2010 Aug 18. J Lipid Res. 2010. PMID: 20719759 Free PMC article.
-
Ethanol and C2 ceramide activate fatty acid oxidation in human hepatoma cells.Sci Rep. 2018 Aug 27;8(1):12923. doi: 10.1038/s41598-018-31025-0. Sci Rep. 2018. PMID: 30150688 Free PMC article.
-
The mechanisms by which lipids coordinately regulate the formation of the protein and lipid domains of the stratum corneum: Role of fatty acids, oxysterols, cholesterol sulfate and ceramides as signaling molecules.Dermatoendocrinol. 2011 Apr;3(2):113-8. doi: 10.4161/derm.3.2.14996. Epub 2011 Apr 1. Dermatoendocrinol. 2011. PMID: 21695021 Free PMC article.
References
-
- Feingold K. R. (2007) J. Lipid Res. 48, 2531–2546 - PubMed
-
- Elias P. M., Feingold K. R. (ed) (2006) Epidermal Lamellar Body as A Multifunctional Secretory Organelle, pp. 261–272, Taylor & Francis, New York
-
- Grayson S., Johnson-Winegar A. G., Wintroub B. U., Isseroff R. R., Epstein E. H., Jr., Elias P. M. (1985) J. Invest. Dermatol. 85, 289–294 - PubMed
-
- Holleran W. M., Takagi Y., Uchida Y. (2006) FEBS Lett. 580, 5456–5466 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

