The cell end marker protein TeaC is involved in growth directionality and septation in Aspergillus nidulans
- PMID: 19429780
- PMCID: PMC2708464
- DOI: 10.1128/EC.00251-08
The cell end marker protein TeaC is involved in growth directionality and septation in Aspergillus nidulans
Abstract
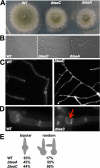
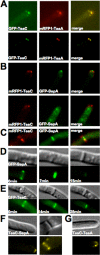
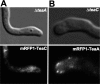
Polarized growth in filamentous fungi depends on the correct spatial organization of the microtubule (MT) and actin cytoskeleton. In Schizosaccharomyces pombe it was shown that the MT cytoskeleton is required for the delivery of so-called cell end marker proteins, e.g., Tea1 and Tea4, to the cell poles. Subsequently, these markers recruit several proteins required for polarized growth, e.g., a formin, which catalyzes actin cable formation. The latest results suggest that this machinery is conserved from fission yeast to Aspergillus nidulans. Here, we have characterized TeaC, a putative homologue of Tea4. Sequence identity between TeaC and Tea4 is only 12.5%, but they both share an SH3 domain in the N-terminal region. Deletion of teaC affected polarized growth and hyphal directionality. Whereas wild-type hyphae grow straight, hyphae of the mutant grow in a zig-zag way, similar to the hyphae of teaA deletion (tea1) strains. Some small, anucleate compartments were observed. Overexpression of teaC repressed septation and caused abnormal swelling of germinating conidia. In agreement with the two roles in polarized growth and in septation, TeaC localized to hyphal tips and to septa. TeaC interacted with the cell end marker protein TeaA at hyphal tips and with the formin SepA at hyphal tips and at septa.
Figures






Similar articles
-
The septin AspB in Aspergillus nidulans forms bars and filaments and plays roles in growth emergence and conidiation.Eukaryot Cell. 2012 Mar;11(3):311-23. doi: 10.1128/EC.05164-11. Epub 2012 Jan 13. Eukaryot Cell. 2012. PMID: 22247265 Free PMC article.
-
The cell-end marker TeaA and the microtubule polymerase AlpA contribute to microtubule guidance at the hyphal tip cortex of Aspergillus nidulans to provide polarity maintenance.J Cell Sci. 2013 Dec 1;126(Pt 23):5400-11. doi: 10.1242/jcs.129841. Epub 2013 Oct 7. J Cell Sci. 2013. PMID: 24101725
-
Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans.Mol Biol Cell. 2008 Jan;19(1):339-51. doi: 10.1091/mbc.e07-06-0523. Epub 2007 Nov 14. Mol Biol Cell. 2008. PMID: 18003978 Free PMC article.
-
Polarized growth in fungi--interplay between the cytoskeleton, positional markers and membrane domains.Mol Microbiol. 2008 May;68(4):813-26. doi: 10.1111/j.1365-2958.2008.06193.x. Epub 2008 Apr 8. Mol Microbiol. 2008. PMID: 18399939 Review.
-
Interdependence of the actin and the microtubule cytoskeleton during fungal growth.Curr Opin Microbiol. 2014 Aug;20:34-41. doi: 10.1016/j.mib.2014.04.005. Epub 2014 May 27. Curr Opin Microbiol. 2014. PMID: 24879477 Review.
Cited by
-
The cell end marker Tea4 regulates morphogenesis and pathogenicity in the basidiomycete fungus Ustilago maydis.Fungal Genet Biol. 2014 May;66:54-68. doi: 10.1016/j.fgb.2014.02.010. Epub 2014 Mar 5. Fungal Genet Biol. 2014. PMID: 24613993 Free PMC article.
-
Dynamics of Actin Cables in Polarized Growth of the Filamentous Fungus Aspergillus nidulans.Front Microbiol. 2016 May 9;7:682. doi: 10.3389/fmicb.2016.00682. eCollection 2016. Front Microbiol. 2016. PMID: 27242709 Free PMC article.
-
The cell-end protein Tea4 spatially regulates hyphal branch initiation and appressorium remodeling in the blast fungus Magnaporthe oryzae.Mol Biol Cell. 2024 Jan 1;35(1):br2. doi: 10.1091/mbc.E23-06-0214. Epub 2023 Oct 30. Mol Biol Cell. 2024. PMID: 37903237 Free PMC article.
-
MoTea4-mediated polarized growth is essential for proper asexual development and pathogenesis in Magnaporthe oryzae.Eukaryot Cell. 2010 Jul;9(7):1029-38. doi: 10.1128/EC.00292-09. Epub 2010 May 14. Eukaryot Cell. 2010. PMID: 20472691 Free PMC article.
-
Identification of interphase functions for the NIMA kinase involving microtubules and the ESCRT pathway.PLoS Genet. 2014 Mar 27;10(3):e1004248. doi: 10.1371/journal.pgen.1004248. eCollection 2014 Mar. PLoS Genet. 2014. PMID: 24675878 Free PMC article.
References
-
- Alvarez-Tabarés, I., A. Grallert, J. M. Ortiz, and I. M. Hagan. 2007. Schizosaccharomyces pombe protein phosphatase 1 in mitosis, endocytosis and a partnership with Wsh3/Tea4 to control polarized growth. J. Cell Sci. 1203589-3601. - PubMed
-
- Basu, R., and F. Chang. 2007. Shaping the actin cytoskeleton using microtubule tips. Curr. Opin. Cell Biol. 191-7. - PubMed
-
- Browning, H., D. D. Hackney, and P. Nurse. 2003. Targeted movement of cell end factors in fission yeast. Nat. Cell Biol. 5812-818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

