The effect of continuous glucose monitoring in well-controlled type 1 diabetes
- PMID: 19429875
- PMCID: PMC2713649
- DOI: 10.2337/dc09-0108
The effect of continuous glucose monitoring in well-controlled type 1 diabetes
Abstract
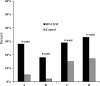
OBJECTIVE The potential benefits of continuous glucose monitoring (CGM) in the management of adults and children with well-controlled type 1 diabetes have not been examined. RESEARCH DESIGN AND METHODS A total of 129 adults and children with intensively treated type 1 diabetes (age range 8-69 years) and A1C <7.0% were randomly assigned to either continuous or standard glucose monitoring for 26 weeks. The main study outcomes were time with glucose level < or =70 mg/dl, A1C level, and severe hypoglycemic events. RESULTS At 26 weeks, biochemical hypoglycemia (< or =70 mg/dl) was less frequent in the CGM group than in the control group (median 54 vs. 91 min/day), but the difference was not statistically significant (P = 0.16). Median time with a glucose level < or =60 mg/dl was 18 versus 35 min/day, respectively (P = 0.05). Time out of range (< or =70 or >180 mg/dl) was significantly lower in the CGM group than in the control group (377 vs. 491 min/day, P = 0.003). There was a significant treatment group difference favoring the CGM group in mean A1C at 26 weeks adjusted for baseline (P < 0.001). One or more severe hypoglycemic events occurred in 10 and 11% of the two groups, respectively (P = 1.0). Four outcome measures combining A1C and hypoglycemia data favored the CGM group in comparison with the control group (P < 0.001, 0.007, 0.005, and 0.003). CONCLUSIONS Most outcomes, including those combining A1C and hypoglycemia, favored the CGM group. The weight of evidence suggests that CGM is beneficial for individuals with type 1 diabetes who have already achieved excellent control with A1C <7.0%.
Figures
References
-
- Irvine AA, Cox D, Gonder-Frederick L: Fear of hypoglycemia: relationship to physical and psychological symptoms in patients with insulin-dependent diabetes mellitus. Health Psychol 1992; 11: 135– 138 - PubMed
-
- Cryer PE: Banting Lecture: Hypoglycemia: the limiting factor in the management of IDDM. Diabetes 1994; 43: 1378– 1389 - PubMed
-
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464– 1476 - PubMed
-
- JDRF CGM Study Group JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther 2008; 10: 310– 321 - PubMed

