Pathogenic mechanisms of tooth agenesis linked to paired domain mutations in human PAX9
- PMID: 19429910
- PMCID: PMC2706687
- DOI: 10.1093/hmg/ddp221
Pathogenic mechanisms of tooth agenesis linked to paired domain mutations in human PAX9
Abstract
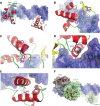
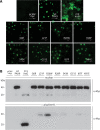
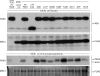
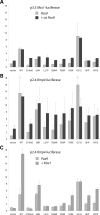
Mutations in the paired-domain transcription factor PAX9 are associated with non-syndromic tooth agenesis that preferentially affects posterior dentition. Of the 18 mutations identified to date, eight are phenotypically well-characterized missense mutations within the DNA-binding paired domain. We determined the structural and functional consequences of these paired domain missense mutations and correlated our findings with the associated dental phenotype variations. In vitro testing included subcellular localization, protein-protein interactions between MSX1 and mutant PAX9 proteins, binding of PAX9 mutants to a DNA consensus site and transcriptional activation from the Pax9 effector promoters Bmp4 and Msx1 with and without MSX1 as co-activator. All mutant PAX9 proteins were localized in the nucleus of transfected cells and physically interacted with MSX1 protein. Three of the mutants retained the ability to bind the consensus paired domain recognition sequence; the others were unable or only partly able to interact with this DNA fragment and also showed a similarly impaired capability for activation of transcription from the Msx1 and Bmp4 promoters. For seven of the eight mutants, the degree of loss of DNA-binding and promoter activation correlated quite well with the severity of the tooth agenesis pattern seen in vivo. One of the mutants however showed neither reduction in DNA-binding nor decrease in transactivation; instead, a loss of responsiveness to synergism with MSX1 in target promoter activation and a dominant negative effect when expressed together with wild-type PAX9 could be observed. Our structure-based studies, which modeled DNA binding and subdomain stability, were able to predict functional consequences quite reliably.
Figures



References
-
- Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 2003;116:1647–1648. - PubMed
-
- Kapadia H., Mues G., D'souza R. Genes affecting tooth morphogenesis. Orthod. Craniofac. Res. 2007;10:237–244. - PubMed
-
- Peters H., Neubuser A., Balling R. Pax genes and organogenesis: Pax9 meets tooth development. Eur. J. Oral Sci. 1998;106(Suppl. 1):38–43. - PubMed
-
- Mostowska A., Biedziak B., Trzeciak W.H. A novel mutation in PAX9 causes familial form of molar oligodontia. Eur. J. Hum. Genet. 2006;14:173–179. - PubMed

