Genetic variation of CYP2C19 affects both pharmacokinetic and pharmacodynamic responses to clopidogrel but not prasugrel in aspirin-treated patients with coronary artery disease
- PMID: 19429918
- PMCID: PMC2709885
- DOI: 10.1093/eurheartj/ehp157
Genetic variation of CYP2C19 affects both pharmacokinetic and pharmacodynamic responses to clopidogrel but not prasugrel in aspirin-treated patients with coronary artery disease
Abstract
Aims: The metabolic pathways leading to the formation of prasugrel and clopidogrel active metabolites differ. We hypothesized that decreased CYP2C19 activity affects the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel.
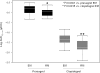
Methods and results: Ninety-eight patients with coronary artery disease (CAD) taking either clopidogrel 600 mg loading dose (LD)/75 mg maintenance dose (MD) or prasugrel 60 mg LD/10 mg MD were genotyped for variation in six CYP genes. Based on CYP genotype, patients were segregated into two groups: normal function (extensive) metabolizers (EM) and reduced function metabolizers (RM). Plasma active metabolite concentrations were measured at 30 min, 1, 2, 4, and 6 h post-LD and during the MD period on Day 2, Day 14, and Day 29 at 30 min, 1, 2, and 4 h. Vasodilator-stimulated phosphoprotein (VASP) and VerifyNow P2Y12 were measured predose, 2, and 24 +/- 4 h post-LD and predose during the MD period on Day 14 +/- 3 and Day 29 +/- 3. For clopidogrel, active metabolite exposure was significantly lower (P = 0.0015) and VASP platelet reactivity index (PRI, %) and VerifyNow P2Y(12) reaction unit (PRU) values were significantly higher (P < 0.05) in the CYP2C19 RM compared with the EM group. For prasugrel, there was no statistically significant difference in active metabolite exposure or pharmacodynamic response between CYP2C19 EM and RM. Variation in the other five genes demonstrated no statistically significant differences in pharmacokinetic or pharmacodynamic responses.
Conclusion: Variation in the gene encoding CYP2C19 in patients with stable CAD contributes to reduced exposure to clopidogrel's active metabolite and a corresponding reduction in P2Y(12) inhibition, but has no significant influence on the response to prasugrel.
Figures

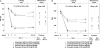
Similar articles
-
The influence of smoking status on the pharmacokinetics and pharmacodynamics of clopidogrel and prasugrel: the PARADOX study.J Am Coll Cardiol. 2013 Aug 6;62(6):505-12. doi: 10.1016/j.jacc.2013.03.037. Epub 2013 Apr 16. J Am Coll Cardiol. 2013. PMID: 23602770 Clinical Trial.
-
Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease.Eur Heart J. 2008 Jan;29(1):21-30. doi: 10.1093/eurheartj/ehm545. Epub 2007 Nov 30. Eur Heart J. 2008. PMID: 18055486 Clinical Trial.
-
Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel.J Thromb Haemost. 2007 Dec;5(12):2429-36. doi: 10.1111/j.1538-7836.2007.02775.x. Epub 2007 Sep 26. J Thromb Haemost. 2007. PMID: 17900275
-
Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans.J Clin Pharmacol. 2010 Feb;50(2):126-42. doi: 10.1177/0091270009343005. Epub 2009 Nov 30. J Clin Pharmacol. 2010. PMID: 19948947 Review.
-
Latest evidence in personalized antiplatelet therapy in patients with acute coronary syndromes undergoing percutaneous coronary intervention.Hosp Pract (1995). 2012 Apr;40(2):104-17. doi: 10.3810/hp.2012.04.976. Hosp Pract (1995). 2012. PMID: 22615085 Review.
Cited by
-
Pharmacogenetics to guide cardiovascular drug therapy.Nat Rev Cardiol. 2021 Sep;18(9):649-665. doi: 10.1038/s41569-021-00549-w. Epub 2021 May 5. Nat Rev Cardiol. 2021. PMID: 33953382 Free PMC article. Review.
-
Antithrombotic therapy in patients with acute coronary syndrome and diabetes mellitus.Herz. 2012 May;37(3):264-72. doi: 10.1007/s00059-012-3610-4. Herz. 2012. PMID: 22456988 Review.
-
Safety of proton pump inhibitors: current evidence for osteoporosis and interaction with antiplatelet agents.Curr Gastroenterol Rep. 2010 Jun;12(3):167-74. doi: 10.1007/s11894-010-0103-6. Curr Gastroenterol Rep. 2010. PMID: 20425476 Review.
-
The association between CYP2C19 genotype and of in-stent restenosis among patients with vertebral artery stent treatment.CNS Neurosci Ther. 2014 Feb;20(2):125-30. doi: 10.1111/cns.12173. Epub 2013 Dec 12. CNS Neurosci Ther. 2014. PMID: 24330577 Free PMC article.
-
Pharmacogenetics of Antiplatelet Therapy.Annu Rev Pharmacol Toxicol. 2023 Jan 20;63:211-229. doi: 10.1146/annurev-pharmtox-051921-092701. Epub 2022 Jan 8. Annu Rev Pharmacol Toxicol. 2023. PMID: 35914768 Free PMC article. Review.
References
-
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505–1516. - PubMed
-
- Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pearson T, Pfeffer MA, Taubert KA. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47:2130–2139. - PubMed
-
- Jakubowski JA, Winters KJ, Naganuma H, Wallentin L. Prasugrel: a novel thienopyridine antiplatelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev. 2007;25:357–374. - PubMed
-
- Savi P, Herbert JM. Clopidogrel and ticlopidine: P2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis. Semin Thromb Haemost. 2005;31:174–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

