Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms
- PMID: 19430489
- PMCID: PMC2704983
- DOI: 10.1038/nm.1958
Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms
Abstract
Inflammation and oxidative stress are pathogenic mediators of many diseases, but molecules that could be therapeutic targets remain elusive. Inflammation and matrix degradation in the vasculature are crucial for abdominal aortic aneurysm (AAA) formation. Cyclophilin A (CypA, encoded by Ppia) is highly expressed in vascular smooth muscle cells (VSMCs), is secreted in response to reactive oxygen species (ROS) and promotes inflammation. Using the angiotensin II (AngII)-induced AAA model in Apoe-/- mice, we show that Apoe-/-Ppia-/- mice are completely protected from AngII-induced AAA formation, in contrast to Apoe-/-Ppia+/+ mice. Apoe-/-Ppia-/- mice show decreased inflammatory cytokine expression, elastic lamina degradation and aortic expansion. These features were not altered by reconstitution of bone marrow cells from Ppia+/+ mice. Mechanistic studies showed that VSMC-derived intracellular and extracellular CypA are required for ROS generation and matrix metalloproteinase-2 activation. These data define a previously undescribed role for CypA in AAA formation and suggest CypA as a new target for treating cardiovascular disease.
Figures

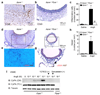
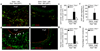
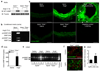
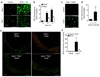

References
-
- Kunieda T, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. - PubMed
-
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. - PubMed
-
- Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. - PubMed
-
- Sun J, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

