Involvment of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons
- PMID: 19430525
- PMCID: PMC2675062
- DOI: 10.1371/journal.pone.0005491
Involvment of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons
Abstract
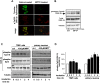
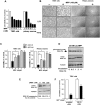
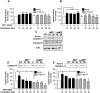
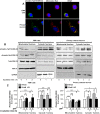
Aberrant mitochondrial function appears to play a central role in dopaminergic neuronal loss in Parkinson's disease (PD). 1-methyl-4-phenylpyridinium iodide (MPP(+)), the active metabolite of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), is a selective inhibitor of mitochondrial complex I and is widely used in rodent and cell models to elicit neurochemical alterations associated with PD. Recent findings suggest that Glycogen Synthase Kinase-3beta (GSK-3beta), a critical activator of neuronal apoptosis, is involved in the dopaminergic cell death. In this study, the role of GSK-3beta in modulating MPP(+)-induced mitochondrial dysfunction and neuronal death was examined in vivo, and in two neuronal cell models namely primary cultured and immortalized neurons. In both cell models, MPTP/MPP(+) treatment caused cell death associated with time- and concentration-dependent activation of GSK-3beta, evidenced by the increased level of the active form of the kinase, i.e. GSK-3beta phosphorylated at tyrosine 216 residue. Using immunocytochemistry and subcellular fractionation techniques, we showed that GSK-3beta partially localized within mitochondria in both neuronal cell models. Moreover, MPP(+) treatment induced a significant decrease of the specific phospho-Tyr216-GSK-3beta labeling in mitochondria concomitantly with an increase into the cytosol. Using two distinct fluorescent probes, we showed that MPP(+) induced cell death through the depolarization of mitochondrial membrane potential. Inhibition of GSK-3beta activity using well-characterized inhibitors, LiCl and kenpaullone, and RNA interference, prevented MPP(+)-induced cell death by blocking mitochondrial membrane potential changes and subsequent caspase-9 and -3 activation. These results indicate that GSK-3beta is a critical mediator of MPTP/MPP(+)-induced neurotoxicity through its ability to regulate mitochondrial functions. Inhibition of GSK-3beta activity might provide protection against mitochondrial stress-induced cell death.
Conflict of interest statement
Figures
References
-
- de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. - PubMed
-
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. - PubMed
-
- Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, et al. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989;163:1450–1455. - PubMed
-
- Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26:719–723. - PubMed
-
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

