Empirical correlation of triggered activity and spatial and temporal re-entrant substrates with arrhythmogenicity in a murine model for Jervell and Lange-Nielsen syndrome
- PMID: 19430811
- PMCID: PMC2719739
- DOI: 10.1007/s00424-009-0671-1
Empirical correlation of triggered activity and spatial and temporal re-entrant substrates with arrhythmogenicity in a murine model for Jervell and Lange-Nielsen syndrome
Abstract
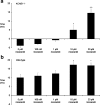
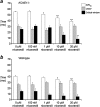
KCNE1 encodes the beta-subunit of the slow component of the delayed rectifier K(+) current. The Jervell and Lange-Nielsen syndrome is characterized by sensorineural deafness, prolonged QT intervals, and ventricular arrhythmogenicity. Loss-of-function mutations in KCNE1 are implicated in the JLN2 subtype. We recorded left ventricular epicardial and endocardial monophasic action potentials (MAPs) in intact, Langendorff-perfused mouse hearts. KCNE1 (-/-) but not wild-type (WT) hearts showed not only triggered activity and spontaneous ventricular tachycardia (VT), but also VT provoked by programmed electrical stimulation. The presence or absence of VT was related to the following set of criteria for re-entrant excitation for the first time in KCNE1 (-/-) hearts: Quantification of APD(90), the MAP duration at 90% repolarization, demonstrated alterations in (1) the difference, APD(90), between endocardial and epicardial APD(90) and (2) critical intervals for local re-excitation, given by differences between APD(90) and ventricular effective refractory period, reflecting spatial re-entrant substrate. Temporal re-entrant substrate was reflected in (3) increased APD(90) alternans, through a range of pacing rates, and (4) steeper epicardial and endocardial APD(90) restitution curves determined with a dynamic pacing protocol. (5) Nicorandil (20 microM) rescued spontaneous and provoked arrhythmogenic phenomena in KCNE1 (-/-) hearts. WTs remained nonarrhythmogenic. Nicorandil correspondingly restored parameters representing re-entrant criteria in KCNE1 (-/-) hearts toward values found in untreated WTs. It shifted such values in WT hearts in similar directions. Together, these findings directly implicate triggered electrical activity and spatial and temporal re-entrant mechanisms in the arrhythmogenesis observed in KCNE1 (-/-) hearts.
Figures



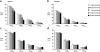



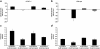
Similar articles
-
Murine Electrophysiological Models of Cardiac Arrhythmogenesis.Physiol Rev. 2017 Jan;97(1):283-409. doi: 10.1152/physrev.00007.2016. Physiol Rev. 2017. PMID: 27974512 Free PMC article. Review.
-
Mechanisms of ventricular arrhythmogenesis in mice following targeted disruption of KCNE1 modelling long QT syndrome 5.J Physiol. 2007 Jan 1;578(Pt 1):99-114. doi: 10.1113/jphysiol.2006.118133. Epub 2006 Nov 9. J Physiol. 2007. PMID: 17095567 Free PMC article.
-
Arrhythmogenic substrate and its modification by nicorandil in a murine model of long QT type 3 syndrome.Prog Biophys Mol Biol. 2008 Oct-Nov;98(2-3):267-80. doi: 10.1016/j.pbiomolbio.2009.01.006. Epub 2009 Jan 24. Prog Biophys Mol Biol. 2008. PMID: 19351517
-
Effects of L-type Ca2+ channel antagonism on ventricular arrhythmogenesis in murine hearts containing a modification in the Scn5a gene modelling human long QT syndrome 3.J Physiol. 2007 Jan 1;578(Pt 1):85-97. doi: 10.1113/jphysiol.2006.121921. Epub 2006 Nov 16. J Physiol. 2007. PMID: 17110414 Free PMC article.
-
[Jervell and Lange-Nielsen syndrome].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019 Sep;33(9):825-829. doi: 10.13201/j.issn.1001-1781.2019.09.007. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019. PMID: 31446697 Review. Chinese.
Cited by
-
Ventricular arrhythmogenesis following slowed conduction in heptanol-treated, Langendorff-perfused mouse hearts.J Physiol Sci. 2012 Mar;62(2):79-92. doi: 10.1007/s12576-011-0187-2. Epub 2012 Jan 5. J Physiol Sci. 2012. PMID: 22219003 Free PMC article.
-
Nicorandil stimulates a Na⁺/Ca²⁺ exchanger by activating guanylate cyclase in guinea pig cardiac myocytes.Pflugers Arch. 2016 Apr;468(4):693-703. doi: 10.1007/s00424-015-1763-8. Epub 2015 Dec 3. Pflugers Arch. 2016. PMID: 26631169
-
Cardiac arrhythmogenesis: roles of ion channels and their functional modification.Front Physiol. 2024 Mar 4;15:1342761. doi: 10.3389/fphys.2024.1342761. eCollection 2024. Front Physiol. 2024. PMID: 38505707 Free PMC article. Review.
-
Murine Electrophysiological Models of Cardiac Arrhythmogenesis.Physiol Rev. 2017 Jan;97(1):283-409. doi: 10.1152/physrev.00007.2016. Physiol Rev. 2017. PMID: 27974512 Free PMC article. Review.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0022-0736(98)90042-5', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0022-0736(98)90042-5'}, {'type': 'PubMed', 'value': '9588657', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9588657/'}]}
- Aizawa Y, Uchiyama H, Yamaura M, Nakayama T, Arita M (1998) Effects of the ATP-sensitive K channel opener nicorandil on the QT interval and the effective refractory period in patients with congenital long QT syndrome. J Electrocardiol 31:117–123 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1540-8159.2006.00507.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1540-8159.2006.00507.x'}, {'type': 'PMC', 'value': 'PMC1978482', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1978482/'}, {'type': 'PubMed', 'value': '17038146', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17038146/'}]}
- Antzelevitch C (2006) Brugada syndrome. Pacing Clin Electrophysiol 29:1130–1159 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1365-2796.2005.01587.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1365-2796.2005.01587.x'}, {'type': 'PMC', 'value': 'PMC1474026', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1474026/'}, {'type': 'PubMed', 'value': '16336513', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16336513/'}]}
- Antzelevitch C, Oliva A (2006) Amplification of spatial dispersion of repolarization underlies sudden cardiac death associated with catecholaminergic polymorphic VT, long QT, short QT and Brugada syndromes. J Intern Med 259:48–58 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1113/jphysiol.2003.048249', 'is_inner': False, 'url': 'https://doi.org/10.1113/jphysiol.2003.048249'}, {'type': 'PMC', 'value': 'PMC2343378', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2343378/'}, {'type': 'PubMed', 'value': '14561835', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/14561835/'}]}
- Balasubramaniam R, Grace AA, Saumarez RC, Vandenberg JI, Huang CLH (2003) Electrogram prolongation and nifedipine-suppressible ventricular arrhythmias in mice following targeted disruption of KCNE1. J Physiol 552:535–546 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/384078a0', 'is_inner': False, 'url': 'https://doi.org/10.1038/384078a0'}, {'type': 'PubMed', 'value': '8900282', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8900282/'}]}
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G (1996) KvLQT1 and IsK (minK) proteins associate to form the IKS cardiac potassium current. Nature 384:78–80 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

