The efficacy and safety of epidermal growth factor in treatment of diabetic foot ulcers: the preliminary results
- PMID: 19432666
- PMCID: PMC7951377
- DOI: 10.1111/j.1742-481X.2009.00594.x
The efficacy and safety of epidermal growth factor in treatment of diabetic foot ulcers: the preliminary results
Abstract
Objective: To evaluate the efficacy and safety of recombinant human epidermal growth factor (rh-EGF) in healing foot ulcers in diabetic patients.
Methods: A total of 28 subjects with foot ulcers were recruited into the pilot study. Patients who had obvious peripheral arterial disease, trans-tibial amputation, plastic surgery or skin flap, and skin graft were excluded. The properly debrided wounds and the non closure wounds after toe amputation were included. When the wounds became clean or uninfected, they received twice-a-day treatment with 0.005% Easyef and hydrocolloid dressing. The size and severity of the wounds were evaluated. Others such as blood sugar, renal and hepatic function, serum albumin, vascular condition, foot infection or osteomyelitis were assessed.
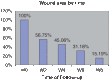
Results: All of 28 patients had positive response of granulation (100%). Complete healing was noted in 13 out of 23 subjects and finished 8-week follow-up (56.5%). The rates of wound closure were 43.3%, 59.9%, 68.7%, and 84.8% in week 2, 4, 6 and 8, respectively, regardless of the severity. Being dropped out, three patients needed further interventions. No skin allergic reaction. Over-granulation was observed in one female patient (3.7%), but as minor.
Conclusions: Easyef has positive effects on healing of moderate-to-severe foot ulcers and demonstrated being safe to diabetic patients. The drug had high tolerability and compliance.
Figures



References
-
- Hoa LT, Han Chau NH. Patient profile in Department of Endocrinology, ChoRay Hospital, 1996–2000. Fulltext of scientific presentations in The first National Conference on Endocrinology and Diabetes, Hanoi, Oct 2001:419–25.
-
- Steed DL. Modulating wound healing in diabetes, The diabetic foot, 6th edn. Missouri USA: Mosby, 2001;395–403.
-
- Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcer. British Jour of Surg 2003;90:133–46. - PubMed
-
- Brown GL, Curtsinger L. Stimulating of healing of chronic wounds by epidermal growth factor. Plast Recontr Surg 1991;88:189–94. - PubMed
-
- Kim KJY. Efficacy and safety of recombinant human epidermal growth factor (rh‐EGF) in the treatment of chronic neuropathic diabetic foot ulcer. Poster 98, 5th International Symposium on the DF; 2007 May 9–12; The Netherlands.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

