Biogenesis of bacterial membrane vesicles
- PMID: 19432795
- PMCID: PMC2745257
- DOI: 10.1111/j.1365-2958.2009.06731.x
Biogenesis of bacterial membrane vesicles
Abstract
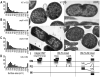
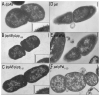
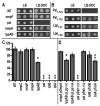
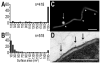
Membrane vesicle (MV) release remains undefined, despite its conservation among replicating Gram-negative bacteria both in vitro and in vivo. Proteins identified in Salmonella MVs, derived from the envelope, control MV production via specific defined domains that promote outer membrane protein-peptidoglycan (OM-PG) and OM protein-inner membrane protein (OM-PG-IM) interactions within the envelope structure. Modulation of OM-PG and OM-PG-IM interactions along the cell body and at division septa, respectively, maintains membrane integrity while co-ordinating localized release of MVs with distinct size distribution and protein content. These data support a model of MV biogenesis, wherein bacterial growth and division invoke temporary, localized reductions in the density of OM-PG and OM-PG-IM associations within the envelope structure, thus releasing OM as MVs.
Figures


References
-
- Aizawa SI, Kubori T. Bacterial flagellation and cell division. Genes Cells. 1998;3:625–634. - PubMed
-
- Alaniz RC, Cummings LA, Bergman MA, Rassoulian-Barrett SL, Cookson BT. Salmonella typhimurium coordinately regulates FliC location and reduces dendritic cell activation and antigen presentation to CD4+ T cells. J Immunol. 2006;177:3983–3993. - PubMed
-
- Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692–7701. - PubMed
-
- Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

