Hepatoma-derived growth factor and its role in keloid pathogenesis
- PMID: 19432814
- PMCID: PMC3828849
- DOI: 10.1111/j.1582-4934.2009.00779.x
Hepatoma-derived growth factor and its role in keloid pathogenesis
Abstract
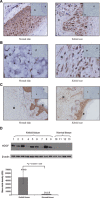
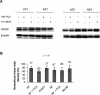
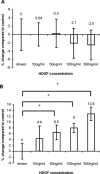
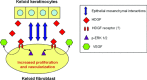
Hepatoma-derived growth factor (HDGF) is a novel mitogenic growth factor that has been implicated in many different carcinomas. Its role in keloid biology has not yet been investigated. The present study is aimed at examining the role of HDGF in keloid pathogenesis. Immunohistochemical staining and Western blot analyses were used to examine in vivo localization and expression of HDGF in keloid and normal skin tissue. This was followed by the detection of HDGF expression in fibroblasts cultured in vitro and fibroblasts exposed to serum. To investigate the effect of epithelial-mesenchymal interactions, a two-chamber system was employed in which keratinocytes on membrane inserts were co-cultured with the fibroblasts. HDGF expression levels in all cell extracts and conditioned media were assayed through Western blot analysis. In another set of experiments, the effect of exogenous recombinant HDGF on keloid fibroblasts (KF) and normal fibroblasts (NF) was examined. Cell proliferation was assessed by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and by quantifying proliferating cell nuclear antigen (PCNA) expression. Downstream targets of HDGF were identified by detecting their expression through Western blot analysis. Our results indicate that there was an increase in HDGF expression in the dermis of keloid compared with normal skin tissue. The application of serum and epithelial-mesenchymal interactions did not seem to have any effect on intracellular HDGF expression levels. However, co-culturing keloid keratinocytes with KFs resulted in increased HDGF secretion when compared with monoculture or normal controls. Furthermore, treatment with exogenous recombinant HDGF was found to increase the proliferation of KFs, activate the extracellular signal-regulated kinase (ERK) pathway and up-regulate the secretion of vascular endothelial growth factor (VEGF).
Figures


Similar articles
-
Epithelial-mesenchymal interactions in keloid pathogenesis modulate vascular endothelial growth factor expression and secretion.J Pathol. 2007 Jan;211(1):95-108. doi: 10.1002/path.2081. J Pathol. 2007. PMID: 17136757
-
Smad3 signalling plays an important role in keloid pathogenesis via epithelial-mesenchymal interactions.J Pathol. 2005 Oct;207(2):232-42. doi: 10.1002/path.1826. J Pathol. 2005. PMID: 16052471
-
IGFBP-7 secreted by adipose-derived stem cells inhibits keloid formation via the BRAF/MEK/ERK signaling pathway.J Dermatol Sci. 2023 Jul;111(1):10-19. doi: 10.1016/j.jdermsci.2023.05.004. Epub 2023 May 19. J Dermatol Sci. 2023. PMID: 37316358
-
Unveiling the Link: The Potential Roles of Vitamin D in Keloid Pathophysiology.Exp Dermatol. 2025 Feb;34(2):e70043. doi: 10.1111/exd.70043. Exp Dermatol. 2025. PMID: 39895409 Free PMC article. Review.
-
Cytokines, chemokines and growth factors involved in keloids pathogenesis.An Bras Dermatol. 2025 Mar-Apr;100(2):300-307. doi: 10.1016/j.abd.2024.01.010. Epub 2025 Jan 10. An Bras Dermatol. 2025. PMID: 39799030 Free PMC article. Review.
Cited by
-
Hepatoma-derived growth factor: a novel prognostic biomarker in intrahepatic cholangiocarcinoma.Tumour Biol. 2015 Jan;36(1):353-64. doi: 10.1007/s13277-014-2651-0. Epub 2014 Sep 28. Tumour Biol. 2015. PMID: 25262276
-
Global research status of pathological scar reported over the period 2001-2021: A 20-year bibliometric analysis.Int Wound J. 2023 May;20(5):1725-1738. doi: 10.1111/iwj.13988. Epub 2022 Oct 23. Int Wound J. 2023. PMID: 36274191 Free PMC article. Review.
-
The Keloid Disorder: Heterogeneity, Histopathology, Mechanisms and Models.Front Cell Dev Biol. 2020 May 26;8:360. doi: 10.3389/fcell.2020.00360. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32528951 Free PMC article. Review.
-
Expression Profile of Long Noncoding RNAs in Human Earlobe Keloids: A Microarray Analysis.Biomed Res Int. 2016;2016:5893481. doi: 10.1155/2016/5893481. Epub 2016 Dec 22. Biomed Res Int. 2016. PMID: 28101509 Free PMC article.
-
Anti-HDGF Antibody Targets EGFR Tyrosine Kinase Inhibitor-Tolerant Cells in NSCLC Patient-Derived Xenografts.Cancer Res Commun. 2024 Sep 1;4(9):2308-2319. doi: 10.1158/2767-9764.CRC-24-0020. Cancer Res Commun. 2024. PMID: 39041204 Free PMC article.
References
-
- Nakamura H, Kambe H, Egawa T, et al. Partial purification and characterization of human hepatoma-derived growth factor. Clin Chim Acta. 1989;183:273–84. - PubMed
-
- Nakamura H, Izumoto Y, Kambe H, et al. Molecular cloning of complementary DNA for a novel human hepatoma-derived growth factor. Its homology with high mobility group-1 protein. J Biol Chem. 1994;269:25143–9. - PubMed
-
- Izumoto Y, Kuroda T, Harada H, et al. Hepatoma-derived growth factor belongs to a gene family in mice showing significant homology in the amino terminus. Biochem Biophys Res Commun. 1997;238:26–32. - PubMed
-
- Everett AD, Stoops T, McNamara CA. Nuclear targeting is required for hepatoma-derived growth factor-stimulated mitogenesis in vascular smooth muscle cells. J Biol Chem. 2001;276:37564–8. - PubMed
-
- Enomoto H, Yoshida K, Kishima Y, et al. Hepatoma-derived growth factor is highly expressed in developing liver and promotes fetal hepatocyte proliferation. Hepatology. 2002;36:1519–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

