The renin-angiotensin system as a primary cause of polyarteritis nodosa in rats
- PMID: 19432815
- PMCID: PMC3828848
- DOI: 10.1111/j.1582-4934.2009.00778.x
The renin-angiotensin system as a primary cause of polyarteritis nodosa in rats
Abstract
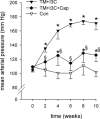
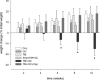
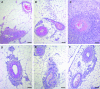
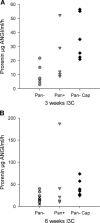
Polyarteritis nodosa is a necrotizing vasculitis of medium-sized arteries of unknown origin. Hypertension is present in 30% of patients with polyarteritis nodosa. In those cases, high renin levels are thought to be secondary to renal involvement. The present study was performed to identify causal factors of polyarteritis nodosa. In cyp1a1ren-2 transgenic rats, vasculitis of medium-sized arteries resembling classical polyarteritis nodosa can be induced. In this model, oral administration of indole-3-carbinol (I3C) activates the liver-specific cyp1a1 promoter, leading to prorenin expression in a dose-dependent manner. After the first 6 weeks of chronic induction with 0.125% I3C, the mean arterial pressure reached a plateau of about 170 mmHg. Ten out of 11 I3C-treated rats, which were chronically instrumented with a telemetric device to measure blood pressure, developed polyarteritis nodosa within 10 weeks of I3C treatment. I3C alone or instrumentation alone did not cause polyarteritis nodosa. The angiotensin-converting enzyme inhibitor captopril completely prevented the development of polyarteritis nodosa, indicating that local angiotensin II generation is a pathogenetic factor in this model. The renin-angiotensin system can play a primary role in the development of polyarteritis nodosa in rats.
Figures



Similar articles
-
Dose-dependent titration of prorenin and blood pressure in Cyp1a1ren-2 transgenic rats: absence of prorenin-induced glomerulosclerosis.J Hypertens. 2008 Jan;26(1):102-9. doi: 10.1097/HJH.0b013e3282f0ab66. J Hypertens. 2008. PMID: 18090546
-
Transient renin-angiotensin system stimulation in an early stage of life causes sustained hypertension in rats.J Hypertens. 2011 Dec;29(12):2369-80. doi: 10.1097/HJH.0b013e32834cfcf4. J Hypertens. 2011. PMID: 22025236
-
Different mechanisms of acute versus long-term antihypertensive effects of soluble epoxide hydrolase inhibition: studies in Cyp1a1-Ren-2 transgenic rats.Clin Exp Pharmacol Physiol. 2014 Dec;41(12):1003-13. doi: 10.1111/1440-1681.12310. Clin Exp Pharmacol Physiol. 2014. PMID: 25224811 Free PMC article.
-
[The changing face of medium-sized vasculitis].Wiad Lek. 2018;71(1 pt 1):64-72. Wiad Lek. 2018. PMID: 29558354 Review. Polish.
-
Polyarteritis nodosa in association with subarachnoid hemorrhage.Intern Med. 2006;45(9):655-8. doi: 10.2169/internalmedicine.45.1632. Epub 2006 Jun 1. Intern Med. 2006. PMID: 16755099 Review.
Cited by
-
Breeding Characteristics and Dose-dependent Blood Pressure Responses of Transgenic Cyp1a1-Ren2 Rats.Comp Med. 2018 Oct 1;68(5):360-366. doi: 10.30802/AALAS-CM-17-18000026. Epub 2018 Sep 5. Comp Med. 2018. PMID: 30185285 Free PMC article.
-
Lack of cardiac fibrosis in a new model of high prorenin hyperaldosteronism.Am J Physiol Heart Circ Physiol. 2009 Nov;297(5):H1845-52. doi: 10.1152/ajpheart.01135.2008. Epub 2009 Sep 11. Am J Physiol Heart Circ Physiol. 2009. PMID: 19749160 Free PMC article.
-
The effect of spironolactone on cardiac and renal fibrosis following myocardial infarction in established hypertension in the transgenic Cyp1a1Ren2 rat.PLoS One. 2021 Nov 29;16(11):e0260554. doi: 10.1371/journal.pone.0260554. eCollection 2021. PLoS One. 2021. PMID: 34843581 Free PMC article.
-
Stress-reactive rats (high-avoidance female rats) have a shorter lifespan than stress-nonreactive rats (low-avoidance female rats).J Toxicol Pathol. 2016 Apr;29(2):77-84. doi: 10.1293/tox.2015-0045. Epub 2015 Dec 7. J Toxicol Pathol. 2016. PMID: 27182111 Free PMC article.
-
Myocardial global longitudinal strain: An early indicator of cardiac interstitial fibrosis modified by spironolactone, in a unique hypertensive rat model.PLoS One. 2019 Aug 12;14(8):e0220837. doi: 10.1371/journal.pone.0220837. eCollection 2019. PLoS One. 2019. PMID: 31404095 Free PMC article.
References
-
- Guillevin L, Mahr A, Callard P, et al. Hepatitis B virus-associated polyarteritis nodosa: clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine. 2005;84:313–22. - PubMed
-
- Bourgarit A, Le Toumelin P, Pagnoux C, et al. Deaths occurring during the first year after treatment onset for polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: a retrospective analysis of causes and factors predictive of mortality based on 595 patients. Medicine. 2005;84:323–30. - PubMed
-
- Cohen L, Guillevin L, Meyrier A, et al. Malignant arterial hypertension in periarteritis nodosa. Incidence, clinicobiologic parameters and prognosis based on a series of 165 cases. Arch Mal Coeur Vaiss. 1986;79:773–8. - PubMed
-
- Graham PC, Lindop GB. The renin-secreting cell in polyarteritis-an immunocytochemical study. Histopathology. 1990;16:339–45. - PubMed
-
- Pintar TJ, Zimmerman S. Hyperreninemic hypertension secondary to a subcapsular perinephric hematoma in a patient with polyarteritis nodosa. Am J Kidney Dis. 1998;32:503–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

