Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase
- PMID: 19432822
- PMCID: PMC3822754
- DOI: 10.1111/j.1582-4934.2009.00771.x
Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase
Abstract
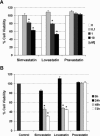
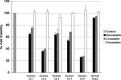
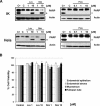
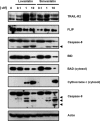
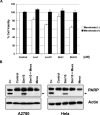
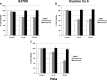
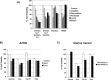
Recent reports have suggested that statins induce cell death in certain epithelial cancers and that patients taking statins to reduce cholesterol levels possess lower cancer incidence. However, little is known about the mechanisms of action of different statins or the effects of these statins in gynaecological malignancies. The apoptotic potential of two lipophilic statins (lovastatin and simvastatin) and one hydrophilic statin (pravastatin) was assessed in cancer cell lines (ovarian, endometrial and cervical) and primary cultured cancerous and normal tissues. Cell viability was studied by MTS assays and apoptosis was confirmed by Western blotting of PARP and flow cytometry. The expressions of key apoptotic cascade proteins were analysed. Our results demonstrate that both lovastatin and simvastatin, but not pravastatin, selectively induced cell death in dose- and time-dependent manner in ovarian, endometrial and cervical cancers. Little or no toxicity was observed with any statin on normal cells. Lipophilic statins induced activation of caspase-8 and -9; BID cleavage, cytochrome C release and PARP cleavage. Statin-sensitive cancers expressed high levels of HMG-CoA reductase compared with resistant cultures. The effect of lipophilic statins was dependent on inhibition of enzymatic activity of HMG-CoA reductase since mevalonate pre-incubation almost completely abrogated the apoptotic effect. Moreover, the apoptotic effect involved the inhibition of synthesis of geranylgeranyl pyrophosphate rather than farnesyl pyrophosphate. In conclusion, lipophilic but not hydrophilic statins induce cell death through activation of extrinsic and intrinsic apoptotic cascades in cancerous cells from the human female genital tract, which express high levels of HMG-CoA reductase. These results promote further investigation in the use of lipophilic statins as anticancer agents in gynaecological malignancies.
Figures



References
-
- Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95:S161–92. - PubMed
-
- Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95:S105–43. - PubMed
-
- Quinn MA, Benedet JL, Odicino F, et al. Carcinoma of the cervix uteri. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95:S43–103. - PubMed
-
- Beckman JA, Creager MA. The nonlipid effects of statins on endothelial function. Trends Cardiovasc Med. 2006;16:156–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

