Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway
- PMID: 19432902
- PMCID: PMC11159028
- DOI: 10.1111/j.1349-7006.2009.01148.x
Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway
Abstract
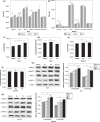
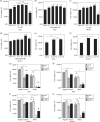
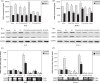
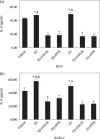
The regulatory mechanism of endometrial carcinoma and the signal transduction pathways involved in hormone action are poorly defined. It has become apparent that the G protein-coupled receptor (GPR) 30 mediates the non-genomic signaling of 17beta-estradiol (E2). Here we show that GPR30 is highly expressed in endometrial cancer tissues and cancer cell lines and positively regulates cell proliferation and invasion. GPR30 expression was detected in 50 human endometrial carcinomas. The transcription level of GPR30 was significantly higher in the tissue of endometrial carcinoma than in normal endometrium (P < 0.05). Immunohistochemical assays revealed that the positive expression rate of GPR30 protein in endometrial carcinoma tissue (35/50, 70%) was statistically higher than in normal endometrium tissue (8/30, 26.67%) (chi2 = 14.16, P = 0.0002). GPR30 overexpression was correlated with high-grade endometrial carcinoma. GPR30 expression was also found in two human endometrial cancer cell lines: RL95-2 (estrogen receptor positive) and KLE (estrogen receptor negative). The roles of GPR30 in proliferative and invasive responses to E2 and G1, a non-steroidal GPR30-specific agonist, in RL95-2 and KLE cell lines were then explored. We showed that E2 and G1 could initiate the MAPK/ERK mitogen-activated protein kinase pathway in both cell lines. What's more, E2 and G1 promoted KLE and RL95-2 proliferation and stimulated matrix metalloproteinase production and activity via the GPR30-mediated MEK/ERK mitogen-activated protein kinase pathway, as well as increased interleukin-6 secretion. These findings suggest that GPR30-mediated non-genomic signaling could play an important role in endometrial cancer.
Figures



Similar articles
-
Estrogenic transmembrane receptor of GPR30 mediates invasion and carcinogenesis by endometrial cancer cell line RL95-2.J Cancer Res Clin Oncol. 2012 May;138(5):775-83. doi: 10.1007/s00432-011-1133-7. Epub 2012 Jan 24. J Cancer Res Clin Oncol. 2012. PMID: 22270964 Free PMC article.
-
The G protein-coupled receptor GPR30 mediates the nontranscriptional effect of estrogen on the activation of PI3K/Akt pathway in endometrial cancer cells.Int J Gynecol Cancer. 2013 Jan;23(1):52-9. doi: 10.1097/IGC.0b013e31827912b8. Int J Gynecol Cancer. 2013. PMID: 23235274
-
Estradiol and tamoxifen induce cell migration through GPR30 and activation of focal adhesion kinase (FAK) in endometrial cancers with low or without nuclear estrogen receptor α (ERα).PLoS One. 2013 Sep 9;8(9):e72999. doi: 10.1371/journal.pone.0072999. eCollection 2013. PLoS One. 2013. PMID: 24039841 Free PMC article.
-
G protein-coupled receptor 30 in tumor development.Endocrine. 2010 Aug;38(1):29-37. doi: 10.1007/s12020-010-9363-z. Epub 2010 Jul 8. Endocrine. 2010. PMID: 20960099 Review.
-
Role of ERβ and GPR30 in the endocrine pancreas: A matter of estrogen dose.Steroids. 2012 Aug;77(10):951-8. doi: 10.1016/j.steroids.2012.01.015. Epub 2012 Jan 28. Steroids. 2012. PMID: 22306576 Review.
Cited by
-
Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology.Endocrinology. 2012 Jul;153(7):2953-62. doi: 10.1210/en.2012-1061. Epub 2012 Apr 11. Endocrinology. 2012. PMID: 22495674 Free PMC article. Review.
-
Oestrogen Inhibits VEGF Expression And Angiogenesis In Triple-Negative Breast Cancer By Activating GPER-1.J Cancer. 2018 Oct 1;9(20):3802-3811. doi: 10.7150/jca.29233. eCollection 2018. J Cancer. 2018. PMID: 30405852 Free PMC article.
-
G protein-coupled receptor 30 mediates cell proliferation of goat mammary epithelial cells via MEK/ERK&PI3K/AKT signaling pathway.Cell Cycle. 2022 Oct;21(19):2027-2037. doi: 10.1080/15384101.2022.2083708. Epub 2022 Jun 6. Cell Cycle. 2022. PMID: 35659445 Free PMC article.
-
G-1 Inhibits Breast Cancer Cell Growth via Targeting Colchicine-Binding Site of Tubulin to Interfere with Microtubule Assembly.Mol Cancer Ther. 2017 Jun;16(6):1080-1091. doi: 10.1158/1535-7163.MCT-16-0626. Epub 2017 Mar 3. Mol Cancer Ther. 2017. PMID: 28258163 Free PMC article.
-
Increased MMPs expression and decreased contraction in the rat myometrium during pregnancy and in response to prolonged stretch and sex hormones.Am J Physiol Endocrinol Metab. 2012 Jul 1;303(1):E55-70. doi: 10.1152/ajpendo.00553.2011. Epub 2012 Apr 10. Am J Physiol Endocrinol Metab. 2012. PMID: 22496348 Free PMC article.
References
-
- Hewitt SC, Deroo BJ, Korach KS. A new mediator for an old hormone? Science 2005; 307: 1572–3. - PubMed
-
- Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nature Rev Cancer 2006; 6: 360–8. - PubMed
-
- Pietras RJ, Levin ER, Szego CM. Estrogen receptors and cell signaling. Science 2005; 310: 51–3. - PubMed
-
- Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science 2003; 301: 1714–17. - PubMed
-
- Revankar CM, Cimino DF, Sklar LA et al . A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005; 307: 1625–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

