Human papillomavirus infections among Japanese women: age-related prevalence and type-specific risk for cervical cancer
- PMID: 19432906
- PMCID: PMC11158131
- DOI: 10.1111/j.1349-7006.2009.01161.x
Human papillomavirus infections among Japanese women: age-related prevalence and type-specific risk for cervical cancer
Abstract
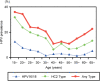
To obtain baseline data for human papillomavirus (HPV) screening and vaccination in Japan, we analyzed HPV DNA data from 2282 Japanese women (1517 normal cytology, 318 cervical intraepithelial neoplasia [CIN] grade 1, 307 CIN2-3, and 140 invasive cervical cancer [ICC]) that visited the University of Tsukuba Hospital or Ibaraki Seinan Medical Center Hospital for screening or treatment of cervical diseases between 1999 and 2007. An L1-based PCR method was used for individual HPV genotyping. The most common HPV types in ICC were, in order of decreasing prevalence, HPV16 (40.5%), HPV18 (24.4%), HPV52 (8.4%), HPV58 (3.1%), and HPV33 (3.1%). Based on the comparison of HPV type distributions between normal cytology and CIN2-3 and ICC, estimated risk of disease progression varied considerably by genotype: HPV16, HPV18, HPV31, HPV33, HPV35, HPV52, and HPV58 (prevalence ratio, 1.92; 95% confidence interval 1.58-2.34); other oncogenic types (0.31, 95% confidence interval 0.19-0.50); and non-oncogenic types (0.09, 95% confidence interval 0.03-0.43). HPV16 and/or HPV18, including coinfections with other types, contributed to 67.1% of ICC and 36.2% of CIN2-3 among Japanese women. More importantly, the overall prevalence of HPV16 and/or HPV18 varied greatly according to the women's age: highest in women aged 20-29 years (ICC, 90.0%; CIN2-3, 53.9%), decreasing with age thereafter, and lowest in women aged 60 years or older (ICC, 56.3%; CIN2-3, 25.0%). In conclusion, type-specific HPV testing may help identify Japanese women at high risk of progression to CIN2-3 and cancer. In Japan, current HPV vaccines are estimated to provide approximately 70% protection against ICC and may be more useful in reducing the incidence of cervical cancer and precancer in young women of reproductive age.
Figures
References
-
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370: 890–907. - PubMed
-
- Wright TC Jr, Schiffman M, Solomon D et al . Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol 2004; 103: 304–9. - PubMed
-
- Future II Study Group . Quadrivalent vaccine against human papillomavirus to prevent high‐grade cervical lesions. N Engl J Med 2007; 356: 1915–27. - PubMed
-
- Harper DM, Franco EL, Wheeler CM et al . Sustained efficacy up to 4.5 years of a bivalent L1 virus‐like particle vaccine against human papillomavirus types 16 and 18: follow‐up from a randomised control trial. Lancet 2006; 367: 1247–55. - PubMed
-
- Paavonen J, Jenkins D, Bosch FX et al . Efficacy of a prophylactic adjuvanted bivalent L1 virus‐like‐particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double‐blind, randomised controlled trial. Lancet 2007; 369: 2161–70. - PubMed

