Binding characteristics of the ovine membrane progesterone receptor alpha and expression of the receptor during the estrous cycle
- PMID: 19432978
- PMCID: PMC2685384
- DOI: 10.1186/1477-7827-7-42
Binding characteristics of the ovine membrane progesterone receptor alpha and expression of the receptor during the estrous cycle
Abstract
Background: Classically, progesterone has been thought to act only through the well-known genomic pathway involving hormone binding to nuclear receptors and subsequent modulation of gene expression. However, there is increasing evidence for rapid, non-genomic effects of progesterone in a variety of mammalian tissues and it is possible that a membrane PR (mPR) is causing these events. We recently isolated and characterized an ovine mPR referred to as mPR-alpha, distinct from the nuclear PR. Based on predicted structural analysis, the ovine mPR-alpha possesses seven transmembrane domains typical of G protein-coupled receptors. Despite the homology to other reported mPRs, information pertaining to the steroid binding characteristics of the ovine mPR-alpha was lacking. Additionally, the ovine mPR-alpha transcript has been identified in the hypothalamus, pituitary, uterus, ovary and corpus luteum, yet changes in expression of the ovine mPR-alpha in these tissues were not known. Consequently, the purpose of this work was to determine the steroid binding characteristics of the ovine mPR-alpha and to investigate possible changes in expression of the ovine mPR-alpha in reproductive tissues throughout the estrous cycle.
Methods: Binding studies were performed using crude membrane fractions from CHO cells expressing the mPR-alpha. Using quantitative Real-time PCR we determined the expression pattern of mRNA for the ovine mPR-alpha during the ovine estrous cycle in tissues known to express the mPR-alpha. Jugular blood samples were also collected and analyzed for serum concentrations of P4 to ensure ewes were at the appropriate stage of their cycle.
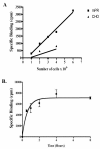
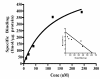
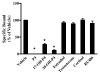
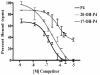
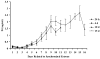
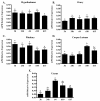
Results: Only progesterone, 20alpha-hydroxyprogesterone and 17alpha-hydroxyprogesterone were able to displace binding of 3H-P4 (P < 0.001) to membrane fractions from CHO cells expressing ovine mPR-alpha. The average B-max and Kd values for three separate experiments were 624 +/- 119 fmol/micro gram protein and 122 +/- 50 nM, respectively. Significant changes in expression of mRNA for the mPR-alpha during the estrous cycle were noted in the corpus luteum and uterus.
Conclusion: The mPR-alpha specifically binds progestins and its expression was correlated to progesterone secretion during the ovine estrous cycle. Results from the present studies suggest that mPR-alpha may have an important physiological role during the ovine estrous cycle.
Figures
Similar articles
-
Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization.Endocrinology. 2006 Sep;147(9):4151-9. doi: 10.1210/en.2006-0002. Epub 2006 Jun 22. Endocrinology. 2006. PMID: 16794007
-
Expression of membrane progestin receptors (mPRs) in the bovine corpus luteum during the estrous cycle and first trimester of pregnancy.Domest Anim Endocrinol. 2018 Apr;63:69-76. doi: 10.1016/j.domaniend.2017.12.004. Epub 2018 Jan 11. Domest Anim Endocrinol. 2018. PMID: 29413904
-
Expression pattern and cellular localization of two critical non-nuclear progesterone receptors in the ovine corpus luteum during the estrous cycle and early pregnancy.Anim Reprod Sci. 2022 Aug;243:107026. doi: 10.1016/j.anireprosci.2022.107026. Epub 2022 Jun 15. Anim Reprod Sci. 2022. PMID: 35752032
-
Non-genomic progesterone receptors in the mammalian ovary: some unresolved issues.Reproduction. 2003 Jan;125(1):3-15. doi: 10.1530/rep.0.1250003. Reproduction. 2003. PMID: 12622691 Review.
-
Establishment of procedures for studying mPR-interacting agents and physiological roles of mPR.Steroids. 2016 Jul;111:79-83. doi: 10.1016/j.steroids.2016.02.015. Epub 2016 Feb 23. Steroids. 2016. PMID: 26917245 Review.
Cited by
-
Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain.Neuroscience. 2011 Jan 13;172:55-65. doi: 10.1016/j.neuroscience.2010.10.051. Epub 2010 Oct 25. Neuroscience. 2011. PMID: 20977928 Free PMC article.
-
Effect of Steroid Hormones, Prostaglandins (E2 and F2α), Oxytocin, and Tumor Necrosis Factor Alpha on Membrane Progesterone (P4) Receptors Gene Expression in Bovine Myometrial Cells.Animals (Basel). 2022 Feb 19;12(4):519. doi: 10.3390/ani12040519. Animals (Basel). 2022. PMID: 35203226 Free PMC article.
-
Progesterone inhibition of oxytocin signaling in endometrium.Front Neurosci. 2013 Aug 7;7:138. doi: 10.3389/fnins.2013.00138. eCollection 2013. Front Neurosci. 2013. PMID: 23966904 Free PMC article.
-
Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications.Steroids. 2011 Jan;76(1-2):11-7. doi: 10.1016/j.steroids.2010.09.006. Epub 2010 Sep 24. Steroids. 2011. PMID: 20869977 Free PMC article. Review.
-
The dilution effect and the importance of selecting the right internal control genes for RT-qPCR: a paradigmatic approach in fetal sheep.BMC Res Notes. 2015 Feb 27;8:58. doi: 10.1186/s13104-015-0973-7. BMC Res Notes. 2015. PMID: 25881111 Free PMC article.
References
-
- Gore-Langton P, Armstrong D. Follicular Steroidogenesis and its Control. In: Knobil E, Neill J, editor. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1988. pp. 331–387.
-
- Niswender GD, Nett TM. The Corpus Luteum and its Control. In: Knobil E, Neill J, editor. The Physiology of Reproduction. Vol. 1. New York: Raven; 1988. pp. 489–526.
-
- Boonyaratanakornkit V, Edwards DP. Receptor mechanisms of rapid extranuclear signalling initiated by steroid hormones. Essays Biochem. 2004;40:105–120. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

