Glimpses of the molecular mechanisms of beta2-microglobulin fibril formation in vitro: aggregation on a complex energy landscape
- PMID: 19433089
- PMCID: PMC2734061
- DOI: 10.1016/j.febslet.2009.05.005
Glimpses of the molecular mechanisms of beta2-microglobulin fibril formation in vitro: aggregation on a complex energy landscape
Abstract
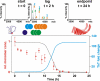
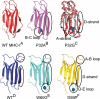
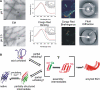
Beta(2)-microglobulin (beta(2)m) is a 99-residue protein that aggregates to form amyloid fibrils in dialysis-related amyloidosis. The protein provides a powerful model for exploration of the structural molecular mechanisms of fibril formation from a full-length protein in vitro. Fibrils have been assembled from beta(2)m under both low pH conditions, where the precursor is disordered, and at neutral pH where the protein is initially natively folded. Here we discuss the roles of sequence and structure in amyloid formation, the current understanding of the structural mechanisms of the early stages of aggregation of beta(2)m at both low and neutral pH, and the common and distinct features of these assembly pathways.
Figures

References
-
- Sipe J.D., Cohen A.S. Review: history of the amyloid fibril. J. Struct. Biol. 2000;130:88–98. - PubMed
-
- Tartaglia G.G., Pawar A.P., Campioni S., Dobson C.M., Chiti F., Vendruscolo M. Prediction of aggregation-prone regions in structured proteins. J. Mol. Biol. 2008;380:425–436. - PubMed
-
- Westermark P. A primer of amyloid nomenclature. Amyloid. 2007;14:179–183. - PubMed
-
- Dobson C.M. Protein folding and misfolding. Nature. 2003;426:884–890. - PubMed
-
- Saper M.A., Bjorkman P.J., Wiley D.C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 Å resolution. J. Mol. Biol. 1991;219:277–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

