Peroxide-induced radical formation at TYR385 and TYR504 in human PGHS-1
- PMID: 19433337
- PMCID: PMC2697118
- DOI: 10.1016/j.jinorgbio.2009.04.002
Peroxide-induced radical formation at TYR385 and TYR504 in human PGHS-1
Abstract
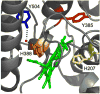
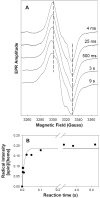
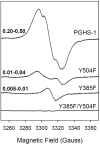
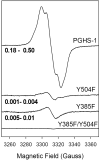
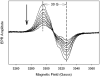
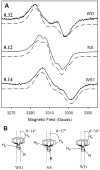
Prostaglandin H synthase isoforms 1 and -2 (PGHS-1 and -2) react with peroxide to form a radical on Tyr385 that initiates the cyclooxygenase catalysis. The tyrosyl radical EPR signals of PGHS-1 and -2 change over time and are altered by cyclooxygenase inhibitor binding. We characterized the tyrosyl radical dynamics using wild type human PGHS-1 (hPGHS-1) and its Y504F, Y385F, and Y385F/Y504F mutants to determine whether the radical EPR signal changes involve Tyr504 radical formation, Tyr385 radical phenyl ring rotation, or both. Reaction of hPGHS-1 with peroxide produced a wide singlet, whereas its Y504F mutant produced only a wide doublet signal, assigned to the Tyr385 radical. The cyclooxygenase specific activity and K(M) value for arachidonate of hPGHS-1 were not affected by the Y504F mutation, but the peroxidase specific activity and the K(M) value for peroxide were increased. The Y385F and Y385F/Y504F mutants retained only a small fraction of the peroxidase activity; the former had a much-reduced yield of peroxide-induced radical and the latter essentially none. After binding of indomethacin, a cyclooxygenase inhibitor, hPGHS-1 produced a narrow singlet but the Y504F mutant did not form a tyrosyl radical. These results indicate that peroxide-induced radicals form on Tyr385 and Tyr504 of hPGHS-1, with radical primarily on Tyr504 in the wild type protein; indomethacin binding prevented radical formation on Tyr385 but allowed radical formation on Tyr504. Thus, hPGHS-1 and -2 have different distributions of peroxide-derived radical between Tyr385 and Tyr504. Y504F mutants in both hPGHS-1 and -2 significantly decreased the cyclooxygenase activation efficiency, indicating that formation of the Tyr504 radical is functionally important for both isoforms.
Figures

Similar articles
-
Identification of Tyr504 as an alternative tyrosyl radical site in human prostaglandin H synthase-2.Biochemistry. 2004 Feb 17;43(6):1560-8. doi: 10.1021/bi035717o. Biochemistry. 2004. PMID: 14769032
-
Role of Tyr348 in Tyr385 radical dynamics and cyclooxygenase inhibitor interactions in prostaglandin H synthase-2.Biochemistry. 2006 Jan 17;45(2):523-32. doi: 10.1021/bi051235w. Biochemistry. 2006. PMID: 16401081 Free PMC article.
-
Structural comparisons of arachidonic acid-induced radicals formed by prostaglandin H synthase-1 and -2.J Inorg Biochem. 2011 Mar;105(3):366-74. doi: 10.1016/j.jinorgbio.2010.11.012. Epub 2010 Nov 27. J Inorg Biochem. 2011. PMID: 21421123 Free PMC article.
-
Prostaglandin H synthase: resolved and unresolved mechanistic issues.Arch Biochem Biophys. 2010 Jan 1;493(1):103-24. doi: 10.1016/j.abb.2009.08.019. Epub 2009 Sep 1. Arch Biochem Biophys. 2010. PMID: 19728984 Free PMC article. Review.
-
Free radical-dependent inhibition of prostaglandin endoperoxide H Synthase-2 by nitro-arachidonic acid.Free Radic Biol Med. 2019 Nov 20;144:176-182. doi: 10.1016/j.freeradbiomed.2019.03.022. Epub 2019 Mar 25. Free Radic Biol Med. 2019. PMID: 30922958 Review.
Cited by
-
Asymmetric acetylation of the cyclooxygenase-2 homodimer by aspirin and its effects on the oxygenation of arachidonic, eicosapentaenoic, and docosahexaenoic acids.Mol Pharmacol. 2010 Jun;77(6):979-86. doi: 10.1124/mol.109.063115. Epub 2010 Mar 1. Mol Pharmacol. 2010. PMID: 20194532 Free PMC article.
-
Cyclooxygenase reaction mechanism of PGHS--evidence for a reversible transition between a pentadienyl radical and a new tyrosyl radical by nitric oxide trapping.J Inorg Biochem. 2011 Mar;105(3):356-65. doi: 10.1016/j.jinorgbio.2010.11.013. J Inorg Biochem. 2011. PMID: 21403766 Free PMC article.
-
A radical transfer pathway in spore photoproduct lyase.Biochemistry. 2013 May 7;52(18):3041-50. doi: 10.1021/bi3016247. Epub 2013 Apr 22. Biochemistry. 2013. PMID: 23607538 Free PMC article.
-
Cyclooxygenase competitive inhibitors alter tyrosyl radical dynamics in prostaglandin H synthase-2.Biochemistry. 2009 Dec 22;48(50):11902-11. doi: 10.1021/bi901600f. Biochemistry. 2009. PMID: 19894761 Free PMC article.
-
Pulsed Dipolar Spectroscopy Reveals That Tyrosyl Radicals Are Generated in Both Monomers of the Cyclooxygenase-2 Dimer.Biochemistry. 2015 Dec 22;54(50):7309-12. doi: 10.1021/acs.biochem.5b00979. Epub 2015 Dec 7. Biochemistry. 2015. PMID: 26636181 Free PMC article.
References
-
- Smith WL, Garavito RM, DeWitt DL. J Biol Chem. 1996;271:33157–33160. - PubMed
-
- Herschman HR. Biochim Biophys Acta. 1996;1299:125–140. - PubMed
-
- Smith WL, Eling TE, Kulmacz RJ, Marnett LJ, Tsai A-L. Biochemistry. 1992;31:3–7. - PubMed
-
- Lambeir AM, Markey CM, Dunford HB, Marnett LJ. J Biol Chem. 1985;260:14894–14896. - PubMed
-
- Dietz R, Nastainczyk W, Ruf HH. Eur J Biochem. 1988;171:321–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

