Transcriptional and translational analysis of biofilm determinants of Aggregatibacter actinomycetemcomitans in response to environmental perturbation
- PMID: 19433550
- PMCID: PMC2708548
- DOI: 10.1128/IAI.00126-09
Transcriptional and translational analysis of biofilm determinants of Aggregatibacter actinomycetemcomitans in response to environmental perturbation
Abstract
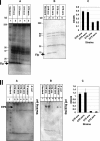
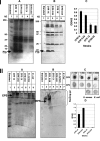
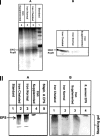
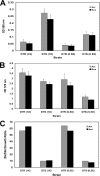
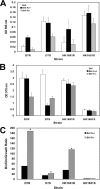
Fimbriae, lipopolysaccharide (LPS), and extracellular polymeric substance (EPS) all contribute to biofilm formation by the periodontopathogen Aggregatibacter actinomycetemcomitans. To understand how individual biofilm determinants respond to changing environmental conditions, the transcription of genes responsible for fimbria, LPS, and EPS production, as well as the translation of these components, was determined in rough (Rv) and isogenic smooth (Sv) variants of A. actinomycetemcomitans cultured in half-strength and full-strength culture medium under anaerobic or aerobic conditions, and in iron-supplemented and iron-chelated medium. The transcription of tadV (fimbrial assembly), pgaC (extracellular polysaccharide synthesis), and orf8 or rmlB (lipopolysaccharide synthesis) was measured by real-time PCR. The amounts of fimbriae, LPS, and EPS were also estimated from stained sodium dodecyl sulfate-polyacrylamide gels and verified by Western blotting and enzyme-linked immunoadsorbent assay using specific antibodies. Each gene was significantly upregulated in the Rv compared to in the Sv. The transcription of fimbrial, LPS, and EPS genes in the Rv was increased approximately twofold in cells cultured in full-strength medium under anaerobic conditions compared to that in cells cultured under aerobic conditions. Under anaerobic conditions, the transcription of fimbrial and EPS enzymes was elevated in both Rv and Sv cells cultured in half-strength medium, compared to that in full-strength medium. Iron chelation also increased the transcription and translation of all biofilm determinants compared to their expression with iron supplementation, yet the quantity of biofilm was not significantly changed by any environmental perturbation except iron limitation. Thus, anaerobic conditions, nutrient stress, and iron limitation each upregulate known biofilm determinants of A. actinomycetemcomitans to contribute to biofilm formation.
Figures

References
-
- Asikainen, S., C. Chen, and J. Slots. 1995. Actinobacillus actinomycetemcomitans genotypes in relation to serotypes and periodontal status. Oral Microbiol. Immunol. 1065-68. - PubMed
-
- Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 1361-92. - PubMed
-
- Camprubí, S., S. Merino, J. F. Guillot, and J. M. Tomas. 1993. The role of the O-antigen lipopolysaccharide on the colonization in vivo of the germfree chicken gut by Klebsiella pneumoniae. Microb. Pathog. 14433-440. - PubMed
-
- Colombo, A. V., C. M. da Silva, A. Haffajee, and A. P. Colombo. 2007. Identification of intracellular oral species within human crevicular epithelial cells from subjects with chronic periodontitis by fluorescence in situ hybridization. J. Periodontal Res. 42236-243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

