Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes
- PMID: 19433586
- PMCID: PMC2709346
- DOI: 10.1074/jbc.M109.008631
Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes
Abstract
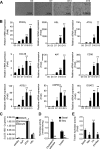
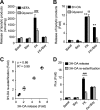
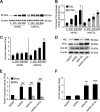
Lipolysis is the catabolic pathway by which triglycerides are hydrolyzed into fatty acids. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) have the capacity to hydrolyze in vitro the first ester bond of triglycerides, but their respective contributions to whole cell lipolysis in human adipocytes is unclear. Here, we have investigated the roles of HSL, ATGL, and its coactivator CGI-58 in basal and forskolin-stimulated lipolysis in a human white adipocyte model, the hMADS cells. The hMADS adipocytes express the various components of fatty acid metabolism and show lipolytic capacity similar to primary cultured adipocytes. We show that lipolysis and fatty acid esterification are tightly coupled except in conditions of stimulated lipolysis. Immunocytochemistry experiments revealed that acute forskolin treatment promotes HSL translocation from the cytosol to small lipid droplets and redistribution of ATGL from the cytosol and large lipid droplets to small lipid droplets, resulting in enriched colocalization of the two lipases. HSL or ATGL overexpression resulted in increased triglyceride-specific hydrolase capacity, but only ATGL overexpression increased whole cell lipolysis. HSL silencing had no effect on basal lipolysis and only partially reduced forskolin-stimulated lipolysis. Conversely, silencing of ATGL or CGI-58 significantly reduced basal lipolysis and essentially abolished forskolin-stimulated lipolysis. Altogether, these results suggest that ATGL/CGI-58 acts independently of HSL and precedes its action in the sequential hydrolysis of triglycerides in human hMADS adipocytes.
Figures



References
-
- Belfrage P., Jergil B., Strålfors P., Tornqvist H. ( 1978) Adv. Exp. Med. Biol. 101, 113– 126 - PubMed
-
- Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T. M., Wagner E. F., Zechner R. ( 2002) J. Biol. Chem. 277, 4806– 4815 - PubMed
-
- Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. ( 2004) Science 306, 1383– 1386 - PubMed
-
- Fredrikson G., Tornqvist H., Belfrage P. ( 1986) Biochim. Biophys. Acta 876, 288– 293 - PubMed
-
- Langin D., Arner P. ( 2006) Trends Endocrinol. Metab. 17, 314– 320 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

