Mechanism of olfactory masking in the sensory cilia
- PMID: 19433623
- PMCID: PMC2713142
- DOI: 10.1085/jgp.200810085
Mechanism of olfactory masking in the sensory cilia
Abstract
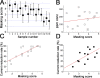
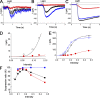
Olfactory masking has been used to erase the unpleasant sensation in human cultures for a long period of history. Here, we show a positive correlation between the human masking and the odorant suppression of the transduction current through the cyclic nucleotide-gated (CNG) and Ca2+-activated Cl- (Cl(Ca)) channels. Channels in the olfactory cilia were activated with the cytoplasmic photolysis of caged compounds, and their sensitiveness to odorant suppression was measured with the whole cell patch clamp. When 16 different types of chemicals were applied to cells, cyclic AMP (cAMP)-induced responses (a mixture of CNG and Cl(Ca) currents) were suppressed widely with these substances, but with different sensitivities. Using the same chemicals, in parallel, we measured human olfactory masking with 6-rate scoring tests and saw a correlation coefficient of 0.81 with the channel block. Ringer's solution that was just preexposed to the odorant-containing air affected the cAMP-induced current of the single cell, suggesting that odorant suppression occurs after the evaporation and air/water partition of the odorant chemicals at the olfactory mucus. To investigate the contribution of Cl(Ca), the current was exclusively activated by using the ultraviolet photolysis of caged Ca, DM-nitrophen. With chemical stimuli, it was confirmed that Cl(Ca) channels were less sensitive to the odorant suppression. It is interpreted, however, that in the natural odorant response the Cl(Ca) is affected by the reduction of Ca2+ influx through the CNG channels as a secondary effect. Because the signal transmission between CNG and Cl(Ca) channels includes nonlinear signal-boosting process, CNG channel blockage leads to an amplified reduction in the net current. In addition, we mapped the distribution of the Cl(Ca) channel in living olfactory single cilium using a submicron local [Ca2+]i elevation with the laser photolysis. Cl(Ca) channels are expressed broadly along the cilia. We conclude that odorants regulate CNG level to express masking, and Cl(Ca) in the cilia carries out the signal amplification and reduction evenly spanning the entire cilia. The present findings may serve possible molecular architectures to design effective masking agents, targeting olfactory manipulation at the nano-scale ciliary membrane.
Figures


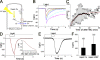







Similar articles
-
Flash photolysis of caged compounds in the cilia of olfactory sensory neurons.J Vis Exp. 2011 Oct 29;(55):e3195. doi: 10.3791/3195. J Vis Exp. 2011. PMID: 22064384 Free PMC article.
-
Single Ca(2+)-activated Cl(-) channel currents recorded from toad olfactory cilia.BMC Neurosci. 2016 Apr 25;17(1):17. doi: 10.1186/s12868-016-0252-0. BMC Neurosci. 2016. PMID: 27113933 Free PMC article.
-
Fast adaptation in mouse olfactory sensory neurons does not require the activity of phosphodiesterase.J Gen Physiol. 2006 Aug;128(2):171-84. doi: 10.1085/jgp.200609555. J Gen Physiol. 2006. PMID: 16880265 Free PMC article.
-
Anoctamin 2/TMEM16B: a calcium-activated chloride channel in olfactory transduction.Exp Physiol. 2012 Feb;97(2):193-9. doi: 10.1113/expphysiol.2011.058230. Epub 2011 Sep 2. Exp Physiol. 2012. PMID: 21890523 Review.
-
Calcium signalling and regulation in olfactory neurons.Curr Opin Neurobiol. 1999 Aug;9(4):419-26. doi: 10.1016/S0959-4388(99)80063-4. Curr Opin Neurobiol. 1999. PMID: 10448159 Review.
Cited by
-
Suppression of olfactory signal transduction by insecticides.NPJ Sci Food. 2019 Jun 3;3:9. doi: 10.1038/s41538-019-0042-z. eCollection 2019. NPJ Sci Food. 2019. PMID: 31304281 Free PMC article.
-
Mixture Coding and Segmentation in the Anterior Piriform Cortex.Front Syst Neurosci. 2020 Nov 19;14:604718. doi: 10.3389/fnsys.2020.604718. eCollection 2020. Front Syst Neurosci. 2020. PMID: 33328914 Free PMC article.
-
Calcium concentration jumps reveal dynamic ion selectivity of calcium-activated chloride currents in mouse olfactory sensory neurons and TMEM16b-transfected HEK 293T cells.J Physiol. 2010 Nov 1;588(Pt 21):4189-204. doi: 10.1113/jphysiol.2010.194407. Epub 2010 Sep 13. J Physiol. 2010. PMID: 20837642 Free PMC article.
-
Phosphoinositide 3-kinase-dependent antagonism in mammalian olfactory receptor neurons.J Neurosci. 2011 Jan 5;31(1):273-80. doi: 10.1523/JNEUROSCI.3698-10.2011. J Neurosci. 2011. PMID: 21209212 Free PMC article.
-
Mediation of mammalian olfactory response by presence of odor-evoked potassium current.Front Allergy. 2024 Oct 16;5:1478529. doi: 10.3389/falgy.2024.1478529. eCollection 2024. Front Allergy. 2024. PMID: 39479387 Free PMC article.
References
-
- Bakalyar H.A., Reed R.R. 1990. Identification of a specialized adenylyl cyclase that may mediate odorant detection.Science. 250:1403–1406 - PubMed
-
- Boccaccio A., Menini A. 2007. Temporal development of cyclic nucleotide-gated and Ca2+-activated Cl- currents in isolated mouse olfactory sensory neurons.J. Neurophysiol. 98:153–160 - PubMed
-
- Brunet L.J., Gold G.H., Ngai J. 1996. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel.Neuron. 4:681–693 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

