Deletion of the secretory vesicle proteins IA-2 and IA-2beta disrupts circadian rhythms of cardiovascular and physical activity
- PMID: 19433624
- PMCID: PMC2735370
- DOI: 10.1096/fj.09-132019
Deletion of the secretory vesicle proteins IA-2 and IA-2beta disrupts circadian rhythms of cardiovascular and physical activity
Abstract
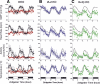
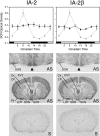
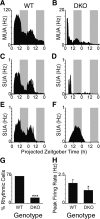
Targeted deletion of IA-2 and IA-2beta, major autoantigens in type 1 diabetes and transmembrane secretory vesicle proteins, results in impaired secretion of hormones and neurotransmitters. In the present study, we evaluated the effect of these deletions on daily rhythms in blood pressure, heart rate, core body temperature, and spontaneous physical and neuronal activity. We found that deletion of both IA-2 and IA-2beta profoundly disrupts the usual diurnal variation of each of these parameters, whereas the deletion of either IA-2 or IA-2beta alone did not produce a major change. In situ hybridization revealed that IA-2 and IA-2beta transcripts are highly but nonrhythmically expressed in the suprachiasmatic nuclei, the site of the brain's master circadian oscillator. Electrophysiological studies on tissue slices from the suprachiasmatic nuclei showed that disruption of both IA-2 and IA-2beta results in significant alterations in neuronal firing. From these studies, we concluded that deletion of IA-2 and IA-2beta, structural proteins of secretory vesicles and modulators of neuroendocrine secretion, has a profound effect on the circadian system.
Figures
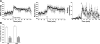

References
-
- Dunlap J C, Loros J J, DeCoursey P J. Sunderland, MA, USA: Sinauer Associates; ChronobiologyBiological Timekeeping. 2004
-
- Reppert S M, Moore R Y, Klein D C. New York.: Oxford University Press; Suprachiasmatic NucleusThe Mind’s Clock. 1991
-
- Brown T M, Piggins H D. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol. 2007;82:229–255. - PubMed
-
- Reppert S M, Weaver D R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

