The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels
- PMID: 19433662
- PMCID: PMC2748878
- DOI: 10.1001/archneurol.2009.48
The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels
Abstract
Objective: To assess the effects of sevoflurane, the most commonly used inhalation anesthetic, on apoptosis and beta-amyloid protein (Abeta) levels in vitro and in vivo. Subjects Naive mice, H4 human neuroglioma cells, and H4 human neuroglioma cells stably transfected to express full-length amyloid precursor protein.
Interventions: Human H4 neuroglioma cells stably transfected to express full-length amyloid precursor protein were exposed to 4.1% sevoflurane for 6 hours. Mice received 2.5% sevoflurane for 2 hours. Caspase-3 activation, apoptosis, and Abeta levels were assessed.
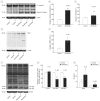
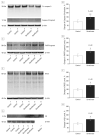
Results: Sevoflurane induced apoptosis and elevated levels of beta-site amyloid precursor protein-cleaving enzyme and Abeta in vitro and in vivo. The caspase inhibitor Z-VAD decreased the effects of sevoflurane on apoptosis and Abeta. Sevoflurane-induced caspase-3 activation was attenuated by the gamma-secretase inhibitor L-685,458 and was potentiated by Abeta. These results suggest that sevoflurane induces caspase activation which, in turn, enhances beta-site amyloid precursor protein-cleaving enzyme and Abeta levels. Increased Abeta levels then induce further rounds of apoptosis.
Conclusions: These results suggest that inhalational anesthetic sevoflurane may promote Alzheimer disease neuropathogenesis. If confirmed in human subjects, it may be prudent to caution against the use of sevoflurane as an anesthetic, especially in those suspected of possessing excessive levels of cerebral Abeta.
Figures





References
-
- Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122(3):1131–1135. - PubMed
-
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. - PubMed
-
- Gu Y, Misonou H, Sato T, Dohmae N, Takio K, Ihara Y. Distinct intramembrane cleavage of the beta-amyloid precursor protein family resembling gamma-secretase-like cleavage of Notch. J Biol Chem. 2001;276(38):35235–35238. - PubMed
-
- Yu C, Kim SH, Ikeuchi T, et al. Characterization of a presenilin-mediated amyloid precursor protein carboxyl-terminal fragment gamma. Evidence for distinct mechanisms involved in gamma-secretase processing of the APP and Notch1 trans-membrane domains. J Biol Chem. 2001;276(47):43756–43760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

