High antigen levels are the cause of T cell exhaustion during chronic viral infection
- PMID: 19433785
- PMCID: PMC2688997
- DOI: 10.1073/pnas.0809818106
High antigen levels are the cause of T cell exhaustion during chronic viral infection
Abstract
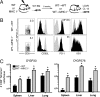
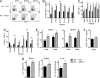
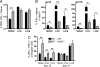
Many persistent viral infections induce dysfunctional T cell responses. Although a negative correlation exists between viral load and T cell responses during chronic infection, it is not known whether high antigen levels are the cause or just the consequence of T cell exhaustion. Furthermore, it is unclear what role antigen presentation by bone-marrow (BM) derived versus infected parenchymal cells has on T cell exhaustion. To address these issues, we examined the influence of antigen presentation by different cell types on CD8(+) T cell responses during persistent infection of mice with lymphocytic choriomeningitis virus (LCMV) clone 13. We generated BM chimeric mice, in which non-BM derived cells were MHC class I deficient. Virus-specific CD8(+) T cells in lymphoid and nonlymphoid tissues were increased in both number and ability to produce cytokines in these mice soon after infection. However, viral clearance from infected MHC I(-/-) parenchyma was significantly impaired, despite increased populations of cytokine producing CTL. The CD8(+) T cell response was overwhelmed by sustained antigen persistence, becoming increasingly exhausted within 4-6 weeks. Thus, we find that (i) sustained antigen presentation directly drives T cell exhaustion during a chronic viral infection, (ii) CTL require direct antigen-MHC interactions to clear virus-infected cells, and (iii) persistent interactions with antigen presented on both hematopoietic and nonhematopoietic cells negatively impacts virus-specific T cell responses during chronic infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Klenerman P, Hill A. T cells and viral persistence: Lessons from diverse infections. Nat Immunol. 2005;6:873–879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

