ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization
- PMID: 19433799
- PMCID: PMC2689030
- DOI: 10.1073/pnas.0900850106
ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization
Abstract
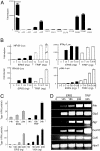
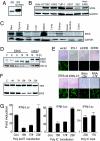
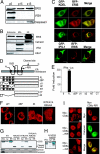
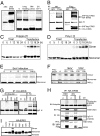
We report here the identification and characterization of a protein, ERIS, an endoplasmic reticulum (ER) IFN stimulator, which is a strong type I IFN stimulator and plays a pivotal role in response to both non-self-cytosolic RNA and dsDNA. ERIS (also known as STING or MITA) resided exclusively on ER membrane. The ER retention/retrieval sequence RIR was found to be critical to retain the protein on ER membrane and to maintain its integrity. ERIS was dimerized on innate immune challenges. Coumermycin-induced ERIS dimerization led to strong and fast IFN induction, suggesting that dimerization of ERIS was critical for self-activation and subsequent downstream signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kumagai Y, Takeuchi O, Akira S. Pathogen recognition by innate receptors. J Infect Chemother. 2008;14:86–92. - PubMed
-
- Yoneyama M, Fujita T. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity. 2008;29:178–181. - PubMed
-
- Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. - PubMed
-
- Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

