Cumulative incidence of false-positive results in repeated, multimodal cancer screening
- PMID: 19433838
- PMCID: PMC2682972
- DOI: 10.1370/afm.942
Cumulative incidence of false-positive results in repeated, multimodal cancer screening
Abstract
Purpose: Multiple cancer screening tests have been advocated for the general population; however, clinicians and patients are not always well-informed of screening burdens. We sought to determine the cumulative risk of a false-positive screening result and the resulting risk of a diagnostic procedure for an individual participating in a multimodal cancer screening program.
Methods: Data were analyzed from the intervention arm of the ongoing Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, a randomized controlled trial to determine the effects of prostate, lung, colorectal, and ovarian cancer screening on disease-specific mortality. The 68,436 participants, aged 55 to 74 years, were randomized to screening or usual care. Women received serial serum tests to detect cancer antigen 125 (CA-125), transvaginal sonograms, posteroanterior-view chest radiographs, and flexible sigmoidoscopies. Men received serial chest radiographs, flexible sigmoidoscopies, digital rectal examinations, and serum prostate-specific antigen tests. Fourteen screening examinations for each sex were possible during the 3-year screening period.
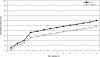
Results: After 14 tests, the cumulative risk of having at least 1 false-positive screening test is 60.4% (95% CI, 59.8%-61.0%) for men, and 48.8% (95% CI, 48.1%-49.4%) for women. The cumulative risk after 14 tests of undergoing an invasive diagnostic procedure prompted by a false-positive test is 28.5% (CI, 27.8%-29.3%) for men and 22.1% (95% CI, 21.4%-22.7%) for women.
Conclusions: For an individual in a multimodal cancer screening trial, the risk of a false-positive finding is about 50% or greater by the 14th test. Physicians should educate patients about the likelihood of false positives and resulting diagnostic interventions when counseling about cancer screening.
Trial registration: ClinicalTrials.gov NCT00002540.
Figures




References
-
- Lung Cancer Alliance. Lung cancer screening and early detection. http://www.lungcanceralliance.org/facing/screening.html. Accessed Apr 24, 2008.
-
- American Urological Association. Prostate cancer screening. http://www.urologyhealth.org/adult/index.cfm?cat=05&topic=250. Accessed Apr 24, 2008.
-
- Centers for Disease Control and Prevention. Colorectal (colon) cancer. Screening guidelines. http://www.cdc.gov/cancer/colorectal/basic_info/screening/guidelines.htm. Accessed Apr 24, 2008.
-
- Armstrong JT, Brandt K, Read Y. Women’s Health Screening in Napa Valley: Uterine, Tube, and Ovarian Screening. http://www.womenshealthscreening.com/pelvic.htm. Accessed Apr 24, 2008.
-
- American Cancer Society. American Cancer Society guidelines for the early detection of cancer. http://www.cancer.org/doc-root/PED/content/PED_2_3X_ACS_Cancer_Detection.... Accessed Apr 24, 2008.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
