Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras
- PMID: 19433858
- PMCID: PMC2727406
- DOI: 10.1182/blood-2009-01-199323
Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras
Abstract
Severe combined immunodeficiency (SCID) is a syndrome of diverse genetic cause characterized by profound deficiencies of T, B, and sometimes NK-cell function. Nonablative human leukocyte antigen-identical or rigorously T cell-depleted haploidentical parental bone marrow transplantation (BMT) results in thymus-dependent genetically donor T-cell development in the recipients, leading to long-term survival. We reported previously that normal T-cell numbers, function, and repertoire developed by 3 to 4 months after transplantation in SCID patients, and the repertoire remained highly diverse for the first 10 years after BMT. The T-cell receptor diversity positively correlated with T-cell receptor excision circle levels, a reflection of thymic output. However, the fate of thymic function in SCID patients beyond 10 to 12 years after BMT remained to be determined. In this greater than 25-year follow-up study of 128 patients with 11 different molecular types of SCID after nonconditioned BMT, we provide evidence that T-cell function, thymic output, and T-cell clonal diversity are maintained long-term.
Figures
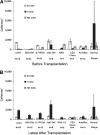

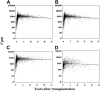


Comment in
-
TRECing long-term success in SCID.Blood. 2009 Aug 13;114(7):1287-8. doi: 10.1182/blood-2009-06-223495. Blood. 2009. PMID: 19679699 No abstract available.
References
-
- Buckley RH, Schiff SE, Schiff RI, et al. Hematopoietic stem cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–516. - PubMed
-
- Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. - PubMed
-
- Buckley RH, Schiff SE, Sampson HA, et al. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986;136:2398–2407. - PubMed
-
- Patel DD, Gooding ME, Parrott RE, et al. Thymic function after hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 2000;342:1325–1332. - PubMed
-
- Sarzotti M, Patel DD, Li X, et al. T cell repertoire development in humans with SCID after nonablative allogeneic marrow transplantation. J Immunol. 2003;170:2711–2718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

