Altered cerebral hemodynamics in early Alzheimer disease: a pilot study using transcranial Doppler
- PMID: 19433892
- PMCID: PMC3210481
- DOI: 10.3233/JAD-2009-1079
Altered cerebral hemodynamics in early Alzheimer disease: a pilot study using transcranial Doppler
Abstract
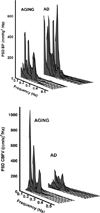
Cerebrovascular disease may contribute to the development and progression of Alzheimer's disease (AD). This study investigated whether impairments in cerebral hemodynamics can be detected in early-stage AD. Nine patients with mild AD and eight cognitively normal controls matched for age underwent brain magnetic resonance imaging and neuropsychological evaluation, followed by assessment of steady-state cerebral blood flow velocity (CBFV, transcranial Doppler), blood pressure (BP, Finapres), and cerebrovascular resistance index (BP/CBFV). Cerebral hemodynamics were quantified using spectral and transfer function analysis of BP and CBFV in rest, during standing up after squat, and during repeated squat-stand maneuvers. Compared to controls, AD patients had lower CBFV and higher cerebrovascular resistance index, unexplained by brain atrophy. Low-frequency variability of BP was enhanced, suggesting impaired arterial baroreflex function. However, CBFV variability was reduced despite enhanced BP variability, and dynamic cerebral autoregulation was not impaired. In conclusion, despite a distinct pattern of altered cerebral hemodynamics, AD patients may have normal autoregulation. However, the challenges for autoregulation in AD are higher, as our data show enhanced BP fluctuations. Increased cerebral vasoconstriction or reduced vasomotion also may attenuate CBFV variability.
Figures



References
-
- Suter OC, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, Miklossy J. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–1992. - PubMed
-
- Aliev G, Smith MA, Obrenovich ME, de la Torre JC, Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Alzheimer disease. Neurotox Res. 2003;5:491–504. - PubMed
-
- Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C. Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab. 2004;24:334–342. - PubMed
-
- Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

