Abnormalities of calcium metabolism and myocardial contractility depression in the failing heart
- PMID: 19434491
- PMCID: PMC2772965
- DOI: 10.1007/s10741-009-9146-x
Abnormalities of calcium metabolism and myocardial contractility depression in the failing heart
Abstract
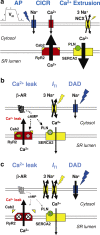
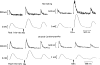
Heart failure (HF) is characterized by molecular and cellular defects which jointly contribute to decreased cardiac pump function. During the development of the initial cardiac damage which leads to HF, adaptive responses activate physiological countermeasures to overcome depressed cardiac function and to maintain blood supply to vital organs in demand of nutrients. However, during the chronic course of most HF syndromes, these compensatory mechanisms are sustained beyond months and contribute to progressive maladaptive remodeling of the heart which is associated with a worse outcome. Of pathophysiological significance are mechanisms which directly control cardiac contractile function including ion- and receptor-mediated intracellular signaling pathways. Importantly, signaling cascades of stress adaptation such as intracellular calcium (Ca(2+)) and 3'-5'-cyclic adenosine monophosphate (cAMP) become dysregulated in HF directly contributing to adverse cardiac remodeling and depression of systolic and diastolic function. Here, we provide an update about Ca(2+) and cAMP dependent signaling changes in HF, how these changes affect cardiac function, and novel therapeutic strategies which directly address the signaling defects.
Figures
References
-
- Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. - PubMed
-
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. - PubMed
-
- Feldman MD, Copelas L, Gwathmey JK, Phillips P, Warren SE, Schoen FJ, Grossman W, Morgan JP. Deficient production of cyclic AMP: pharmacologic evidence of an important cause of contractile dysfunction in patients with end-stage heart failure. Circulation. 1987;75:331–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

