Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory
- PMID: 19434554
- PMCID: PMC2739305
- DOI: 10.1055/s-0029-1216356
Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory
Abstract
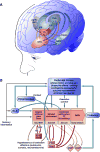
Drug addiction is conceptualized as chronic, relapsing compulsive use of drugs with significant dysregulation of brain hedonic systems. Compulsive drug use is accompanied by decreased function of brain substrates for drug positive reinforcement and recruitment of brain substrates mediating the negative reinforcement of motivational withdrawal. The neural substrates for motivational withdrawal ("dark side" of addiction) involve recruitment of elements of the extended amygdala and the brain stress systems, including corticotropin-releasing factor and norepinephrine. These changes, combined with decreased reward function, are hypothesized to persist in the form of an allostatic state that forms a powerful motivational background for relapse. Relapse also involves a key role for the basolateral amygdala in mediating the motivational effects of stimuli previously paired with drug seeking and drug motivational withdrawal. The basolateral amygdala has a key role in mediating emotional memories in general. The hypothesis argued here is that brain stress systems activated by the motivational consequences of drug withdrawal can not only form the basis for negative reinforcement that drives drug seeking, but also potentiate associative mechanisms that perpetuate the emotional state and help drive the allostatic state of addiction.
Figures



Similar articles
-
Addiction as a stress surfeit disorder.Neuropharmacology. 2014 Jan;76 Pt B(0 0):370-82. doi: 10.1016/j.neuropharm.2013.05.024. Epub 2013 Jun 6. Neuropharmacology. 2014. PMID: 23747571 Free PMC article. Review.
-
Antireward, compulsivity, and addiction: seminal contributions of Dr. Athina Markou to motivational dysregulation in addiction.Psychopharmacology (Berl). 2017 May;234(9-10):1315-1332. doi: 10.1007/s00213-016-4484-6. Epub 2017 Jan 3. Psychopharmacology (Berl). 2017. PMID: 28050629 Review.
-
Neurobiological substrates for the dark side of compulsivity in addiction.Neuropharmacology. 2009;56 Suppl 1(Suppl 1):18-31. doi: 10.1016/j.neuropharm.2008.07.043. Epub 2008 Aug 7. Neuropharmacology. 2009. PMID: 18725236 Free PMC article. Review.
-
Addiction and the brain antireward system.Annu Rev Psychol. 2008;59:29-53. doi: 10.1146/annurev.psych.59.103006.093548. Annu Rev Psychol. 2008. PMID: 18154498 Review.
-
Neuroadaptive mechanisms of addiction: studies on the extended amygdala.Eur Neuropsychopharmacol. 2003 Dec;13(6):442-52. doi: 10.1016/j.euroneuro.2003.08.005. Eur Neuropsychopharmacol. 2003. PMID: 14636960 Review.
Cited by
-
How does stress lead to risk of alcohol relapse?Alcohol Res. 2012;34(4):432-40. Alcohol Res. 2012. PMID: 23584109 Free PMC article. Review.
-
"Death drive" scientifically reconsidered: Not a drive but a collection of trauma-induced auto-addictive diseases.Front Psychol. 2022 Sep 28;13:941328. doi: 10.3389/fpsyg.2022.941328. eCollection 2022. Front Psychol. 2022. PMID: 36248574 Free PMC article.
-
Regulation of NMDA Receptor Plasticity in the BNST Following Adolescent Alcohol Exposure.Front Cell Neurosci. 2019 Oct 4;13:440. doi: 10.3389/fncel.2019.00440. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31636539 Free PMC article.
-
The Association Between Negative and Positive Affect and Alcohol Use: An Ambulatory Study.J Stud Alcohol Drugs. 2019 Nov;80(6):614-622. doi: 10.15288/jsad.2019.80.614. J Stud Alcohol Drugs. 2019. PMID: 31790351 Free PMC article.
-
Silver nanoparticles (Ag-NPs) in the central amygdala protect the rat conditioned by morphine from withdrawal attack due to naloxone via high-level nitric oxide.Naunyn Schmiedebergs Arch Pharmacol. 2020 May;393(5):857-866. doi: 10.1007/s00210-019-01784-2. Epub 2020 Jan 2. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31897505
References
-
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. - PubMed
-
- Alheid GF, De Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 1995. pp. 495–578.
-
- Alling C, Balldin J, Bokstrom K, Gottfries CG, Karlsson I, Langstrom G. Studies on duration of a late recovery period after chronic abuse of ethanol: a cross-sectional study of biochemical and psychiatric indicators. Acta Psychiatr Scand. 1982;66:384–397. - PubMed
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Press; 2000. text revision.
-
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology. 1991;103:227–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA10072/DA/NIDA NIH HHS/United States
- DK26741/DK/NIDDK NIH HHS/United States
- DA04398/DA/NIDA NIH HHS/United States
- AA08459/AA/NIAAA NIH HHS/United States
- R01 AA008459/AA/NIAAA NIH HHS/United States
- P01 DK026741/DK/NIDDK NIH HHS/United States
- P60 AA006420/AA/NIAAA NIH HHS/United States
- DA04043/DA/NIDA NIH HHS/United States
- R01 DA004043/DA/NIDA NIH HHS/United States
- R01 DA004398/DA/NIDA NIH HHS/United States
- P50 AA006420/AA/NIAAA NIH HHS/United States
- AA06420/AA/NIAAA NIH HHS/United States
- R37 AA008459/AA/NIAAA NIH HHS/United States
- R01 DA010072/DA/NIDA NIH HHS/United States

