Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir
- PMID: 19434630
- PMCID: PMC3777615
- DOI: 10.1002/rmv.615
Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir
Abstract
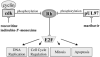
The serine/threonine kinase expressed by human cytomegalovirus from gene UL97 phosphorylates the antiviral drug ganciclovir, but its biological function is the phosphorylation of its natural viral and cellular protein substrates which affect viral replication at many levels. The UL97 kinase null phenotype is therefore complex, as is the mechanism of action of maribavir, a highly specific inhibitor of its enzymatic activity. Studies that utilise the drug corroborate results from genetic approaches and together have elucidated many functions of the UL97 kinase that are critical for viral replication. The kinase phosphorylates eukaryotic elongation factor 1delta, the carboxyl terminal domain of the large subunit of RNA polymerase II, the retinoblastoma tumour suppressor and lamins A and C. Each of these is also phosphorylated and regulated by cdc2/cyclin-dependent kinase 1, suggesting that the viral kinase may perform a similar function. These and other activities of the UL97 kinase appear to stimulate the cell cycle to support viral DNA synthesis, enhance the expression of viral genes, promote virion morphogenesis and facilitate the egress of mature capsids from the nucleus. In the absence of UL97 kinase activity, viral DNA synthesis is inefficient and structural proteins are sequestered in nuclear aggresomes, reducing the efficiency of virion morphogenesis. Mature capsids that do form fail to egress the nucleus as the nuclear lamina are not dispersed by the kinase. The critical functions performed by the UL97 kinase illustrate its importance in viral replication and confirm that the kinase is a target for the development of antiviral therapies.
Figures


References
-
- Cunningham C, Davison AJ, Dolan A, et al. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J Gen Virol. 1992;73(Pt 2):303–311. - PubMed
-
- Overton HA, McMillan DJ, Klavinskis LS, Hope L, Ritchie AJ, Wong-kai-in P. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology. 1992;190(1):184–192. - PubMed
-
- Stevenson D, Colman KL, Davison AJ. Characterization of the putative protein kinases specified by varicella-zoster virus genes 47 and 66. J Gen Virol. 1994;75(Pt 2):317–326. - PubMed
-
- van Zeijl M, Fairhurst J, Baum EZ, Sun L, Jones TR. The human cytomegalovirus UL97 protein is phosphorylated and a component of virions. Virology. 1997;231(1):72–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

